Advancing Drug Delivery at AstraZeneca
Like many companies there has been an increased focus on drug delivery at AstraZeneca over recent years and an explosion of opportunities for delivery science. Traditionally, like many large biopharma companies, drug delivery was focused on delivering small molecule drug candidates which needed enhanced exposure through poorly soluble technologies or prolonged exposure via controlled release technologies. These were used as enabling technologies at launch or for life cycle management and some excellent delivery science was applied to our medicines and capabilities built up in poorly soluble technologies, in particular, amorphous solid dispersions, and pelleted systems for controlled release. Biopharmaceutics and solid-state science grew as a capability to support particularly our oral and inhaled products.
An exception is goserelin acetate, our LHRH agonist, a decapeptide, which was encapsulated within a PLGA implant to prolong its release and provide a monthly sub cutaneous injection. This was first approved in 1989 for prostate cancer and subsequently a 3 monthly injection was approved, and indications expanded to include breast cancer and endometriosis. This remains an important therapy for patients and an example where delivery science and some talented scientists were critical to the development of a new modality. We make our own polymers and have built our own sterile manufacturing plant and dedicated packaging facility to produce the product. Another example where delivery science was critical, was in the development of our estrogen receptor (ER) downregulator, fulvestrant, which is extremely poorly soluble and suffers from first pass metabolism thus precluding its oral dosing. Creative, courageous and tenacious scientists developed a long acting intra-muscular sterile oily injection leading to its first launch in 2002 as a monotherapy for post-menopausal women with breast cancer. There was some challenge about this unusual route of administration for a breast cancer therapy at the time, as well as the patient acceptability of the two 5ml injections required to deliver the dose. However, the combination of formulation science, device engineering, education and an efficacious drug has enabled an important medicine for patients with hormone responsive breast cancer.
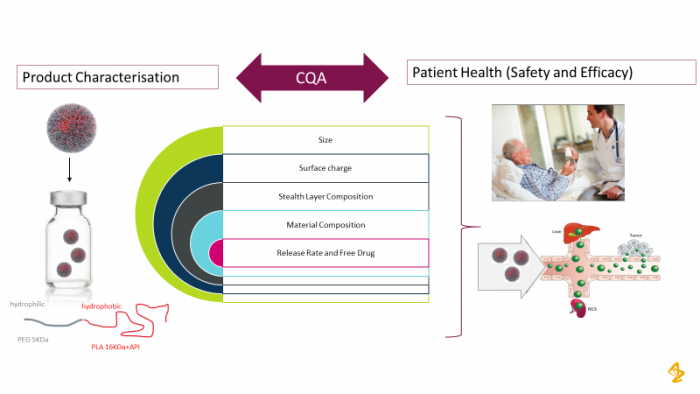
At the same time nearly 10 years ago, an initiative to apply drug delivery science to improve therapeutic index was initiated with the focus mainly on biophysical tissue targeting. It was initiated to provide drug delivery solutions for increased tissue specificity and change the drug distribution in oncology drugs in particular. The effort was initially through a collaboration with BIND Therapeutics using their polymeric nanoparticles and later, with Starpharma with their DEP® Dendrimer technology to improve efficacy or safety and enable the development of two Oncology drug candidates. These are an aurora kinase B inhibitor nanoparticle [1, 2], which is in Phase II development for small cell lung cancer (NCT 04525391) and Phase I/II in hematological cancers (NCT 03217838) and a dual Bcl-2/Bcl xL inhibitor conjugated to a 5th generation pegylated poly-L-lysine dendrimer (NCT 04214093). A lot of emphasis, internally has been placed on the successfully translating of these nanomedicines from both a design and development perspective[3, 4]. Figure 1 illustrates some of the important critical quality attributes that need to be understood to ensure robust in vivo performance. We have proactively participated in and organized a number of panel discussions and Industry Round Tables to learn and collaborate in this space. We have now invested in polymer science internally to give us more flexibility to apply a range of delivery systems to our diverse portfolio ; designing them for versatility and ease of manufacture and pharmaceutical development [5, 6]. This platform is now being used alongside our new Tumour Targeted Delivery Department, in Early Oncology and offers an alternative approach to biological targeting via Antibody Drug Conjugates (ADCs).
Figure 1: Critical Quality Attributes need to be identified to ensure reproducible in vivo performance of nanomedicines
Collaborating with Ionis on antisense oligonucleotides and Moderna on mRNA technology drove a capability build in the delivery science associated with delivery nucleic acid-based therapeutics. This is together with the demand for delivery in order to realize the potential our leading work in therapeutic gene editing. To date, lipid nanoparticles have been the primary focus for intracellular delivery of mRNA [7, 8]. We have also focused on developing capability pharmaceutics, biopharmaceutic and advanced analytical science required for the translation of these modalities [9, 10]. This is a critical aspect to get started on early as the development aspects of such advanced therapeutics may look very different compared to traditional small molecules and biologics [11].
To build knowledge and understanding in intracellular delivery in order to help the design of more efficient delivery systems, a cross discipline intracellular drug delivery group within our Advanced Drug Delivery Department has been set up. Their focus is to aid in the understanding of cell uptake and trafficking, design of new delivery materials and advanced analytical characterization and to work with leading external collaborators in this space. To rapidly screen novel materials and formulations for intracellular delivery, a high throughput design make test cycle has been created to characterize formulations and their effect on cell uptake, cell toxicity, endosomal escape and protein expression.
The drive for better targeting and novel biomaterials for intracellular delivery biomaterials has created a new discipline of the “delivery chemist” which, to date, hasn’t commonly been a role within the Pharmaceutical Industry and has an associated new skill set. This has driven the use of novel lipid, polymers and polypeptides as carrier materials and well as the design novel linkers and conjugation chemistries for ligand attachment ([12-14]
AstraZeneca has also ventured into the field of oral biologics and explored more classical and newer technologies. On the classical side the transient permeation enhancer (TPE) based oral peptide delivery has been the most applied approach in preclinical and clinical studies. The technology has led to two drugs on the market recently, Semaglutide/RYBELSUS® (Novo Nordisk) and Octrotide/MYCAPPSA (Chiasma®). The TPE is expected to disrupt the tight junctions within the gastrointestinal tract enabling transport of the macromolecule across the tight junctions and subsequent absorption. However, this approach has several disadvantages; poor bioavailability, high variability, and food effect as well as developability and operational issues. Other options in the traditional space we considered are nanoparticles, emulsions, intestinal patches or stents and mucoadhesive technologies. These technologies however only give rise to moderate increases in bioavailability.
Delivery of peptides using active delivery technologies, where the absorption barriers are disrupted coupled with immediate micro injection and transfer of the macromolecule, may offer the ability to deliver higher efficacious quantities across the GI mucosa with more acceptable drug product variability and bioavailability. Such technologies have the potential to help us to meet our oral biologics needs and are currently undergoing active investigation.
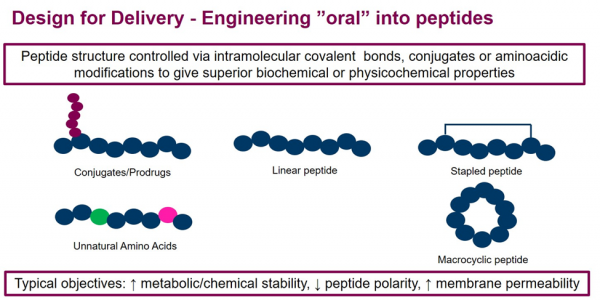
In summary, AstraZeneca has transitioned from classical drug discovery influencing candidate selection from a product perspective to a predominantly design for delivery approach. The concept is illustrated in Figure 2 and exemplified for oral peptides in Figure 3. The increasing diversity of the modalities and the delivery being fundamental to their success has driven this change. Briefly, previously the target was identified, then the modality selected, and finally delivery strategy determined in a serial fashion. The scope of drug delivery was relatively narrow. Now the scope has expanded, and once the target has been selected all modalities and delivery options can be considered in parallel. The complexity of drug delivery has also increased, and more modalities and routes are now in play along with intracellular delivery options.
Figure 2: Transition to a Parallel Approach to Drug Design where Design for Delivery is Critical
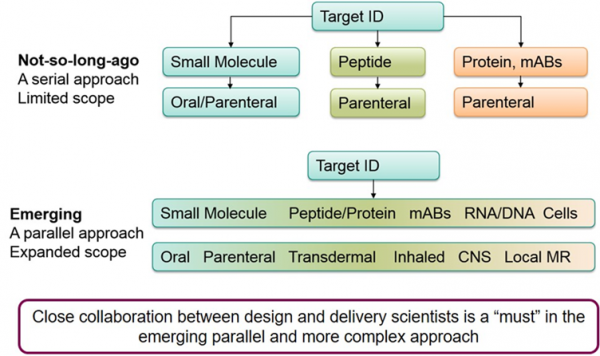
Figure 3: Design for Delivery: Approaches to design peptides for oral delivery
With the strategic ambitions of our therapy areas, progress in understanding disease drivers, diversity of the modalities and progress in delivery science; the Advanced Drug Delivery group in AstraZeneca has quintupled in size over the last 4 years since its inception in 2016. Key to its success has been the translational mission of the department involving coining novel delivery concepts in discovery and translating them into successful clinical products. To do this successfully over a range of modalities, small molecules, amino acid and nucleotide-based therapeutics, the creative scientists has been key. Advanced Drug Delivery has a diverse membership of delivery and formulation scientists, delivery chemists and analytical chemists as well as cell and molecular biologists
This article just touches the surface of delivery science at AstraZeneca, and Advanced Drug Delivery is just one of a few delivery departments in the company. Please see this article for additional details on cool delivery science at AstraZeneca on protein therapeutics, DNA, cell therapies and more. This presents lots of opportunities for talented delivery scientists. Delivery science has changed and is growing [15] and exciting within Industry, and core to the success of next generation therapeutics.
1. Ashton, S., et al., Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci Transl Med, 2016. 8(325): p. 325ra17.
2. Song, Y.H., et al., A novel in situ hydrophobic ion paring (HIP) formulation strategy for clinical product selection of a nanoparticle drug delivery system. J Control Release, 2016. 229: p. 106-119.
3. Hare, J.I., et al., Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev, 2017. 108: p. 25-38.
4. Ashford, M., Development and Commercialization of Nanocarrier‐Based Drug Products, in Pharmaceutical Nanotechnology: Innovation and Production: Innovation and Production, J.C.D.P.A.O.P.A.K.D.P.M.V.d. Voorde, Editor. 2017, Wiley‐VCH Verlag GmbH & Co. KGaA.
5. England, R.M., et al., Tumour regression and improved gastrointestinal tolerability from controlled release of SN-38 from novel polyoxazoline-modified dendrimers. J Control Release, 2017. 247: p. 73-85.
6. England, R.M., et al., Synthesis and Characterization of Dendrimer-Based Polysarcosine Star Polymers: Well-Defined, Versatile Platforms Designed for Drug-Delivery Applications. Biomacromolecules, 2020. 21(8): p. 3332-3341.
7. Yanez Arteta, M., et al., Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc Natl Acad Sci U S A, 2018. 115(15): p. E3351-E3360.
8. Sayers, E.J., et al., Endocytic Profiling of Cancer Cell Models Reveals Critical Factors Influencing LNP-Mediated mRNA Delivery and Protein Expression. Mol Ther, 2019. 27(11): p. 1950-1962.
9. Capaldi, D., et al., Impurities in Oligonucleotide Drug Substances and Drug Products. Nucleic Acid Ther, 2017. 27(6): p. 309-322.
10. Clogston, J.D., et al., Sizing up the Next Generation of Nanomedicines. Pharm Res, 2019. 37(1): p. 6.
11. Bak, A., et al., Translating Cell and Gene Biopharmaceutical Products for Health and Market Impact. Product Scaling From Clinical to Marketplace: Lessons Learned and Future Outlook. J Pharm Sci, 2019. 108(10): p. 3169-3175.
12. Bargh, J.D., et al., Cleavable linkers in antibody-drug conjugates. Chem Soc Rev, 2019. 48(16): p. 4361-4374.
13. St Amant, A.H., et al., A Reactive Antibody Platform for One-Step Production of Antibody-Drug Conjugates through a Diels-Alder Reaction with Maleimide. Bioconjug Chem, 2019. 30(9): p. 2340-2348.
14. Ulkoski, D., et al., Recent advances in polymeric materials for the delivery of RNA therapeutics. Expert Opin Drug Deliv, 2019. 16(11): p. 1149-1167.
15. Ashford, M., Drug delivery-the increasing momentum. Drug Deliv Transl Res, 2020. 10(6): p. 1888-1894.

