Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles
Introduction
Nano-sized materials are widely applied as drug delivery systems because of their ability to distribute in the body and enter cells, and the possibility to engineer them for multiple purposes.1 In recent years, particular interest has been drawn on the impact of the biological environment on nanomaterial interactions with cells. Biomolecules present in the environment in which nanomaterials are applied, such as blood, can adsorb on their surface, leading to the formation of a biomolecular corona, which strongly affects the following outcomes at cell and organism levels.2 Additionally, corona proteins can be recognised by cell receptors. For instance, the uptake of silica nanoparticles is mediated by recognition of corona proteins by the low density lipoprotein receptor, LDLR.3 However, it is not known whether corona composition and the initial recognition of corona proteins by cell receptors also affect the mechanism cells use to internalize the nanoparticles.
To this aim, in this work we characterised and compared the mechanisms of uptake of the same nanoparticles when coated by different coronas, formed by dispersion in different serum concentrations (Figure 1).4 50 nm silica (SiO2) were selected as a model nanoparticle and uptake was studied on cancer epithelial HeLa cells. RNA interference and common pharmacological inhibitors of endocytosis were used to characterize the mechanisms of uptake for the different coronas.
Experimental Methods
Different coronas were prepared on 50 nm silica by incubation with 62 or 12 mg/ml pooled human serum (high and low corona nanoparticles, HC and LC, respectively). The corona-nanoparticle complexes were isolated from the unbound serum biomolecules and characterized by dynamic light scattering (DLS), differential centrifugal sedimentation (DCS) and SDS-PAGE. Corona proteins were identified by mass spectrometry. Then, in order to characterize the mechanism involved, uptake of the corona-nanoparticle complexes by HeLa cells was determined by flow cytometry after silencing the expression of the LDLR or in the presence of a panel of transport inhibitors, using previously optimized conditions.5 For detailed experimental procedures, see Francia et al.4
Results and Discussion
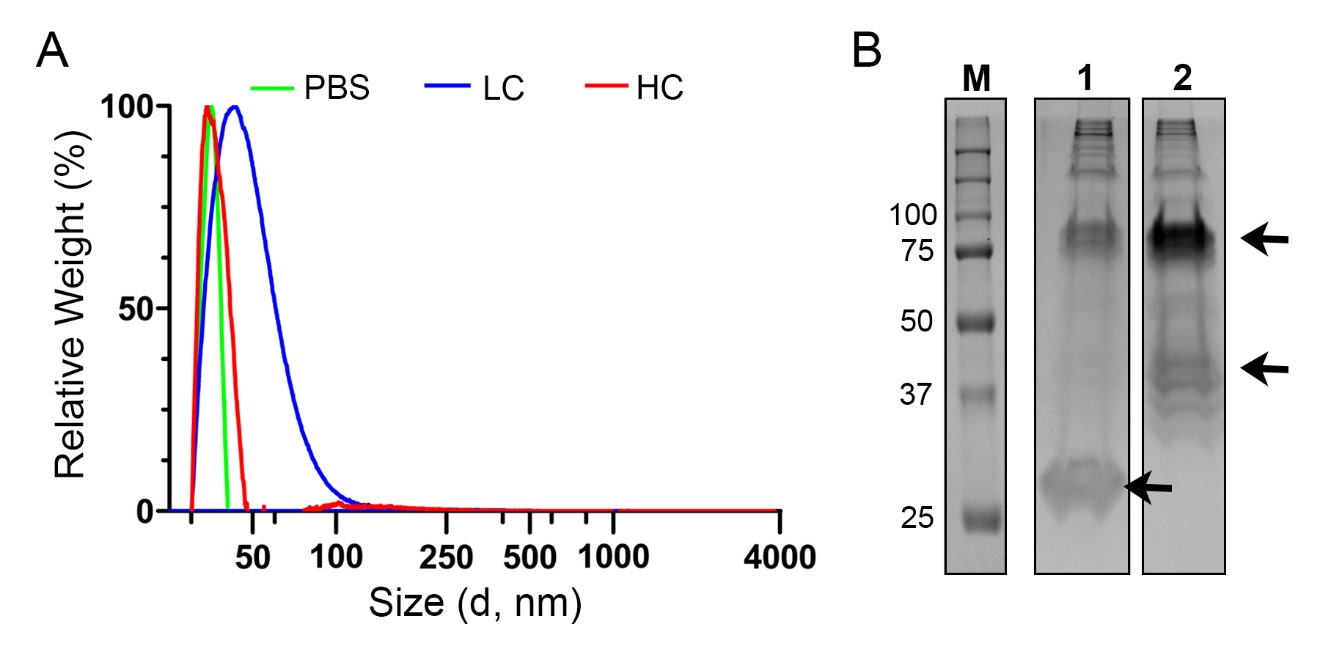
The corona-coated 50 nm SiO2 nanoparticle dispersions formed at different serum concentrations were characterized. DCS results (Figure 2A) excluded the presence of large agglomerates and confirmed that homogenous dispersions of corona-nanoparticle complexes could be obtained, possibly including some dimers in the case of the LC. Dispersion in different serum concentrations led to adsorption of different amounts and types of proteins on the surface of the nanoparticles (Figure 2B). About 300 different proteins were identified by mass spectrometry in both conditions. Although most of the proteins were present in both coronas, the relative abundance of some of them was very different between the two samples (Table 1).
| Gene Name | Protein Name | % of Total | |
|---|---|---|---|
| LC | HC | ||
| APOAI | Apolipoprotein A-I | 17.9 | 7.0 |
| HRG | Histidine-rich glycoprotein | 5.4 | 9.5 |
| SAA4 | Serum amyloid A-4 protein | 4.4 | 2.7 |
| APOE | Apolipoprotein E | 3.9 | 3.2 |
| APOA2 | Apolipoprotein A-II | 3.2 | 1.9 |
| APOB | Apolipoprotein B-100 | 2.6 | 2.2 |
| A1AT | Alpha-1-antitrypsin | 1.7 | 1.5 |
| PON1 | Serum paraoxonase/arylesterase 1 | 1.5 | 0.9 |
| APOA4 | Apolipoprotein A-IV | 1.5 | 1.0 |
| TTHY | Transthyretin | 1.3 | 1.5 |
| ALBU | Serum albumin | 1.3 | 1.3 |
| APOL1 | Apolipoprotein L1 | 1.3 | 0.8 |
| IGKC | Immunoglobulin kappa constant | 1.2 | 1.2 |
| SAA1 | Serum amyloid A-1 protein | 1.2 | 0.7 |
| APOC3 | Apolipoprotein C-III | 1.0 | 0.5 |
Table 1. List of the most abundant proteins identified in the corona of LC and HC complexes. The table shows the relative abundance of the top 15 proteins identified. Readapted from Francia et al.4
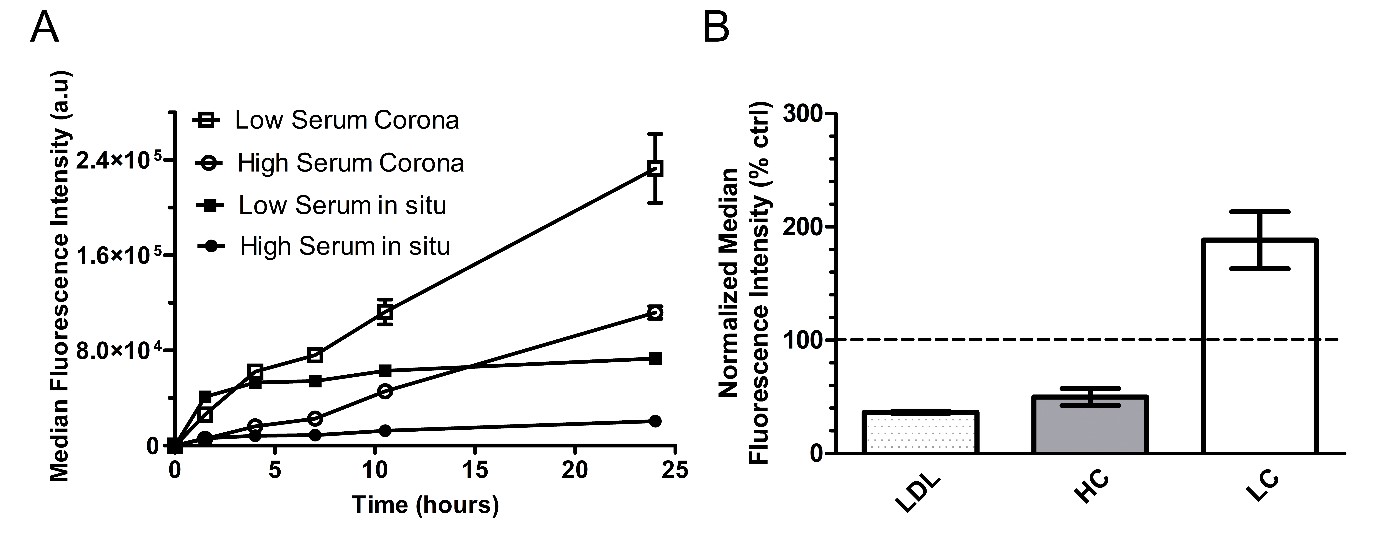
Thus, uptake efficiency was tested on HeLa cells exposed to the nanoparticles in the presence of low and high serum content (4 and 20 mg/ml) or after isolation of the corona-nanoparticle complexes and removal of the free proteins in solution – LC and HC, respectively (Figure 3A). Uptake was lower in high amount of serum, and for both conditions it increased after removal of the free proteins in solution, when the corona-nanoparticle complexes were isolated. However, even after corona isolation, the uptake of the corona-nanoparticle complexes formed in high amount of serum was lower.
Then, we used RNA interference to test the involvement of the LDLR in the recognition of the corona complexes in the two conditions (Figure 3B).3 Interestingly, we found that silencing the LDLR reduced uptake of the HC, but not of LC. Thus, the nanoparticles are recognized differently by cell receptors when covered by different coronas.
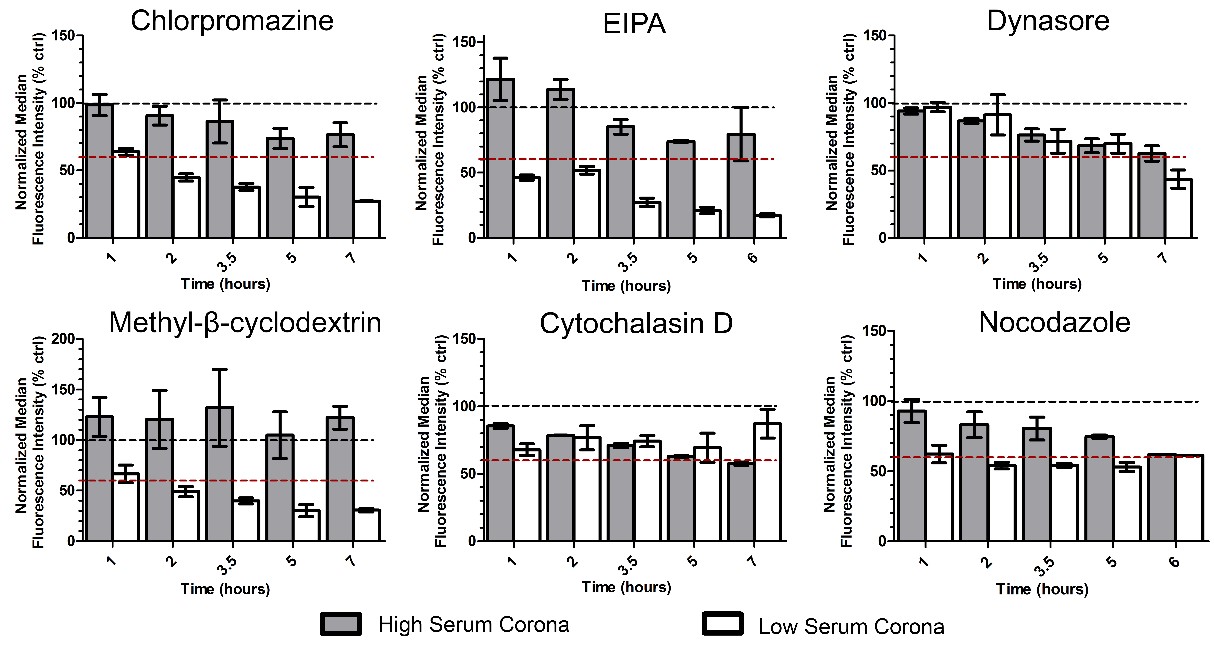
Finally, a panel of six different inhibitors was used to characterize the mechanisms of uptake (Figure 4). Cells were exposed to chlorpromazine (a clathrin-mediated endocytosis inhibitor), EIPA (an inhibitor of macropinocytosis), methyl-β-cyclodextrin (a compound that sequesters the cholesterol in the cell membrane, used to test its involvement in the uptake mechanism), dynasore (inhibitor of dynamin, a key protein for several pathways of endocytosis), cytochalasin D and nocodazole (used, respectively, to block polymerization of the actin cytoskeleton and microtubules).
Overall, the results suggested an involvement of multiple mechanisms in the two conditions and, interestingly, they clearly showed that different mechanisms were involved in the uptake of the silica nanoparticles when coated by a different corona. More in detail, clathrin-mediated endocytosis, macropinocytosis and cholesterol all were involved preferentially in the uptake of LC, while dynamin and microtubules were involved in both conditions but mainly at long exposure times, and actin had stronger effects on HC uptake. Overall, all inhibitors seemed to have minor effects in reducing HC uptake, possibly also connected to the lower uptake of these complexes.
Nevertheless, the results clearly indicated that the mechanisms cells use to internalize nanoparticles are affected by corona composition. Similar observations were reproduced using 200 nm silica and liposomes with different coronas, and also on other cells, including A549 epithelial cancer cells and primary endothelial HUVEC (data not shown).4
Conclusions
Our results showed that the corona composition can affect the initial recognition by cell receptors and, as a result, the same nanoparticles can be internalized by cells using different mechanisms when coated by different coronas.
Another interesting observation is that nanoparticle uptake is lower at higher serum content also after corona isolation and removal of excess free proteins, possibly due to specific differences in corona composition. Next to this, the results suggest that multiple uptake mechanisms seem involved at the same time in the uptake of nanoparticles.
Overall, these results clearly highlight the importance of defining what the “correct corona” is for each nanomedicine (or nanoparticle) when investigating its behaviour on cells, since this layer affects not only recognition by cell receptors, but also the mechanisms cells use for nanoparticle internalization.
Acknowledgments
Text and the figures have been readapted from the ACS Nano article Francia et al. (https://doi.org/10.1021/acsnano.9b03824) with permission from the American Chemical Society. Further permissions related to the material excerpted should be directed to the ACS. This work was funded by the European Research Council (ERC), grant agreement Nº637614 (NanoPaths) to A.S.
References
- Shi, J.; Kantoff, P. W.; Wooster, R.; Farokhzad, O. C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37.
- Monopoli, M. P.; Åberg, C.; Salvati, A.; Dawson, K. A. Biomolecular Coronas Provide the Biological Identity of Nanosized Materials. Nat. Nanotechnol. 2012, 7, 779–786.
- Lara, S.; Alnasser, F.; Polo, E.; Garry, D.; Lo Giudice, M. C.; Hristov, D. R.; Rocks, L.; Salvati, A.; Yan, Y.; Dawson, K. A. Identification of Receptor Binding to the Biomolecular Corona of Nanoparticles. ACS Nano 2017, 11, 1884–1893.
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 10, 11107–11121.
- Francia, V.; Reker-Smit, C.; Boel, G.; Salvati, A. Limits and Challenges in Using Transport Inhibitors to Characterize How Nano-Sized Drug Carriers Enter Cells. Nanomedicine (London, U. K.) 2019, 14, 1533–1549.

