Data-enriched Edible Pharmaceuticals (DEEP) - A novel technology for next generation pharmaceuticals
Natalja Genina - @NataljaGenina
Jukka Rantanen - @JTRantanen
Take a look around – it is basically all of us having a smartphone in our hand. Smartphones have become an integrated part of our daily life and we are almost fully dependent on the smartphone and related apps. It is getting increasingly easy to collect a huge load of personal data related to our health, activity and social contacts. All these data are stored and the related information is used for different purposes, and it offers a huge potential for better patient care. However, classical dosage forms such as tablets have been only at a limited level integrated into this picture. Even the classical pharmaceutical word ‘tablet’ has a dual meaning, and nowadays a simple Google search with the word ‘tablet’ returns nothing else but products from the IT business area.
This ever-changing world with, e.g., old words suddenly possessing new meanings for the general public, is underpinning the need for a change in our way of thinking and talking about pharmaceutical products. Future pharmaceutical products require new features: we should build in personalized elements and include information into the product itself. These products should be easily personalized for an individual dose and there should be a more straightforward solution for combining several compounds into a single product. One should also be able to design the physical appearance of the product based on the patient needs, not based on the limitations that a manufacturing principle dating back from the 19th century is providing. Traditional tablet manufacturing is based on powder handling processes. An alternative approach is to build up the desired product with a desired dose in a fully customized look and functionality using technologies, such as (1) two-dimensional (2D) printing (inkjet printing) , (2) 3D (three-dimensional) printing.
(1) 2D Printing
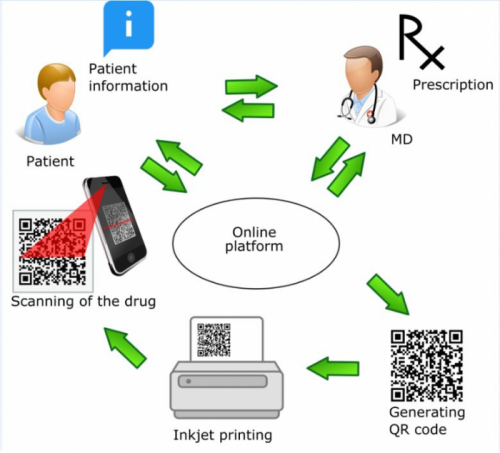
Manufacturing of oral dosage forms containing information-rich 2D patterns, so called data-enriched edible pharmaceuticals (DEEP) that a smartphone equipped with a suitable app can recognize, could be a way to personalize the drug product (Fig. 1).

Fig. 1. Data-enriched edible pharmaceuticals (DEEP), where a quick response (QR) code pattern contains the customized drug dose and at the same time securely encodes relevant information [1].
The drug compound (active pharmaceutical ingredient (API)) can be formulated as an ink and printed in a quick response (QR) coded pattern onto an edible ‘paper’ for oral administration [2]. The ‘edible’ carrier can be in the form of placebo oral film, for example, as Listerine® oral care strips or a more complex carrier system that would allow a good print definition without significant edge bleeding. The production of these QR coded pharmaceuticals can be tailored to fit each patient and has the potential to protect against wrong medication and counterfeit medicine (Fig. 2).
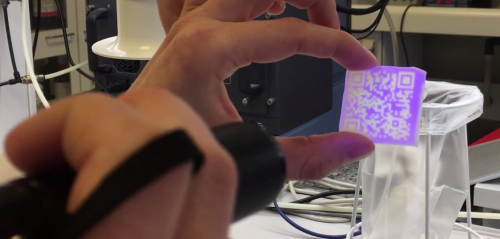
Fig. 2. DEEP with physical anti-counterfeit features [3,4].
DEEP contain the drug itself and information relevant to the patient, healthcare professional, manufacture and/or other relevant information that can be easily read by a smartphone in a secured way. It will ensure that the patient takes the right medication at a right time and in the right way. For example, an alarm or another reminder can be encoded in DEEP for timely administration of the medicine. At the same time, DEEP with security features will guarantee that sensitive information is protected, and the privacy of a patient is maintained.
Link to video https://vimeo.com/253397934
There are already some existing solutions for printing these systems, for example, printers for making cake decoration do exist. However, further optimization and validation of the materials and printers for production of medicine is of current research interest [5]. Screening and optimizing of the drug-containing ink, edible ‘paper’ composition and development of suitable, preferably, non-destructive analytical techniques should be performed to assure the required quality of the printed dosage forms. In future, it could be an opportunity to print on-demand these QR encoded medicines near end-users, for example, at the compounding or hospital pharmacy (Fig. 3). This is not the current practice, so robust and non-destructive innovative quality control tools are needed.
Furthermore, the research should include more functionality in the DEEP based dosage forms, such as addition of both digital and physical anti-counterfeit features to maximize the protection of the medicine from falsification [6]. The specific smartphone applications can be designed and implemented to ensure that the patient has administered the medicine, and by that increasing patient adherence [7]. Future work should also cover related big data challenges – we can collect not only medical information, but also combine this health data with food, lifestyle and social network information. This would be a total paradigm shift on how we think about medicines and pharmaceutical production or pharmaceutical product design.
Fig. 3. A holistic view for implementation of DEEP technology platform with traceable elements in healthcare system. (Reprinted with permission from Edinger et al., 2018. Int. J. Pharm. 536, 138-145).
(2) 3D Printing
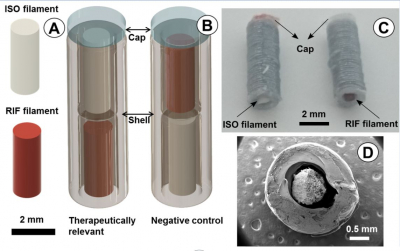
Additive manufacturing opens up a possibility for more innovative product design and allows manufacturing of structures that are not possible using existing, widely-accepted technologies [8,9]. Introducing multi-compartmental systems is a way to accomplish the idea of the innovative by-design products by, for example, 3D printing based on Fused Deposition Modelling (FDM). This technology allows fabrication of customized drug products that could contain different amounts of APIs incorporated into the same dosage unit, a so called ‘polypill’. The unique design of each compartment can be used to determine the release profile of the incorporated API from this specific compartment (Fig. 4). Furthermore, both liquid and solid APIs could be formulated in the same dosage unit, allowing the flexibility in the selection of the combination therapy [10]. Moreover, digital information-rich pattern such as QR codes could also be included on the 3D printed dosage forms in one step process to facilitate the recognition of medicine [4].
Fig. 4. Compartmental dosage forms for controlled oral delivery (Reprinted with permission from Genina et al. 2017. J. Control. Release 268, 40-48).
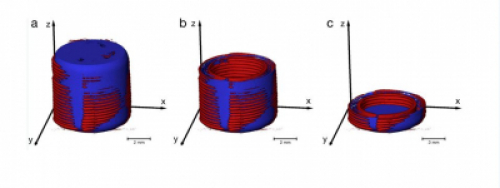
Many aspects of future manufacturing rely on that the additively manufactured dosage form is produced according to a given computer-aided design (a CAD file) and it is subsequently necessary to have analytical techniques capable of ensuring the success of this procedure (Fig. 5). One example of high-end imaging tools is X-ray computed microtomography (XμCT) that has been shown to be capable of doing this [11]. The fast development of diverse imaging modalities is pointing towards real-time release testing of additively manufactured pharmaceutical products [12–15].
Fig.5. The blue colors represent regions where the XμCT data identifies lack of deposition of material according to the CAD model. The red colors represent regions where the XμCT data identifies deposited material that should not have been there according to the CAD model. (Modified from REF: Markl et al. 2017. Pharm. Res. 34, 1037-1052 under the license (http://creativecommons.org/licenses/by/4.0/)).
Summary
Data-enriched edible pharmaceuticals (DEEP) can be implemented into a digital health scenario, where each patient is following the course of the disease with an algorithm-based support. An important feature of these products is that by printing the drug in novel information-rich patterns, both product and patient specific information can be included into the edible end-product. Ultimately, a supply chain based on DEEP based products will enable more precise and safe medication.
References
1. Öblom, H.; Cornett, C.; Bøtker, J.; Frokjaer, S.; Hansen, H.; Rades, T.; Rantanen, J.; Genina, N. Data-enriched edible pharmaceuticals (DEEP) of medical cannabis by inkjet printing. Int. J. Pharm. 2020, 589, 119866, doi:10.1016/j.ijpharm.2020.119866.
2. Oh, B.C.; Jin, G.; Park, C.; Park, J.B.; Lee, B.J. Preparation and evaluation of identifiable quick response (QR)-coded orodispersible films using 3D printer with directly feeding nozzle. Int. J. Pharm. 2020, 584, 119405, doi:10.1016/j.ijpharm.2020.119405.
3. Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm. 2018, 536(1):138-145. doi: 10.1016/j.ijpharm.2017.11.052.
4. Trenfield, S.J.; Xian Tan, H.; Awad, A.; Buanz, A.; Gaisford, S.; Basit, A.W.; Goyanes, A. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. Int. J. Pharm. 2019, 567, 118443, doi:10.1016/j.ijpharm.2019.06.034.
5. Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497, doi:10.1016/j.ijpharm.2019.118497.
6. Leem, J.W.; Kim, M.S.; Choi, S.H.; Kim, S.R.; Kim, S.W.; Song, Y.M.; Young, R.J.; Kim, Y.L. Edible unclonable functions. Nat. Commun. 2020, 11, 1–11, doi:10.1038/s41467-019-14066-5.
7. Nørfeldt, L.; Bøtker, J.; Edinger, M.; Genina, N.; Rantanen, J. Cryptopharmaceuticals: Increasing the Safety of Medication by a Blockchain of Pharmaceutical Products. J. Pharm. Sci. 2019, doi:10.1016/j.xphs.2019.04.025.
8. Rantanen, J.; Khinast, J. The Future of Pharmaceutical Manufacturing Sciences. J. Pharm. Sci. 2015, 104, 3612–3638, doi:10.1002/jps.24594.
9. Ghosh, U.; Ning, S.; Wang, Y.; Kong, Y.L. Addressing Unmet Clinical Needs with 3D Printing Technologies. Adv. Healthc. Mater. 2018, 7, 1800417, doi:10.1002/adhm.201800417.
10. Genina, N.; Boetker, J.P.; Colombo, S.; Harmankaya, N.; Rantanen, J.; Bohr, A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. J. Control. Release 2017, 268, doi:10.1016/j.jconrel.2017.10.003.
11. Markl, D.; Zeitler, J.A.; Rasch, C.; Michaelsen, M.H.; Müllertz, A.; Rantanen, J.; Rades, T.; Bøtker, J. Analysis of 3D prints by X-ray computed microtomography and terahertz pulsed imaging. Pharm. Res. 2017, 34, 1037–1052, doi:10.1007/s11095-016-2083-1.
12. Vakili, H.; Kolakovic, R.; Genina, N.; Marmion, M.; Salo, H.; Ihalainen, P.; Peltonen, J.; Sandler, N. Hyperspectral imaging in quality control of inkjet printed personalised dosage forms. Int. J. Pharm. 2015, 483, 244–249, doi:10.1016/j.ijpharm.2014.12.034.
13. Khorasani, M.; Edinger, M.; Raijada, D.; Bøtker, J.; Aho, J.; Rantanen, J. Near-infrared chemical imaging (NIR-CI) of 3D printed pharmaceuticals. Int. J. Pharm. 2016, 515, 324–330, doi:10.1016/j.ijpharm.2016.09.075.
14. Edinger, M.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Visualization and non-destructive quantification of inkjet-printed pharmaceuticals on different substrates using Raman spectroscopy and Raman chemical imaging. Pharm. Res. 2017, 34, 1023–1036, doi:10.1007/s11095-017-2126-2.
15. Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int. J. Pharm. 2020, 577, 119066, doi:10.1016/j.ijpharm.2020.119066.