An Overview of Coffee Silverskin Validation as a Cosmetic Ingredient
Over the past decades, demand for natural resources has accelerated to the extent that it is now widely considered a serious threat to the proper functioning of economies and societies owing to associated environmental problems such as climate change, biodiversity loss, desertification, and ecosystem degradation.1 At a time when eco-consumerism is increasingly focusing end-user attention on all aspects of products, including raw material sourcing, manufacturing, and disposal, the beauty product sector has been accused of numerous environmentally unfriendly practices.2 Within the rapidly growing market for personal care products, a number of analyses show that the highest environmental impact of cosmetic products is at the consumer level, where consumer concerns range from unsustainable sourcing of raw materials, to pollution (both in the manufacturing phase and the disposal of packaging and products) and animal testing.2 In response, companies have attempted to address these issues holistically by focusing on sustainable raw material sourcing and greener formulations.2 However, these laudable practices face both consumer skepticism, which often views these activities as “green washing,” and the reality of the market, because in a global economy there is little desire for premium pricing to cover the increased costs of these products compared with conventional products. Agro-industrial by-products have the potential to be used for different purposes, providing economic advantage over other disposable residues.3 In particular, the field of skin care products and cosmetics may benefit from these remaining materials.3 Few studies report possible uses for agro-industrial wastes. Human skin is a complex organ that regulates body heat and water loss, while preventing the entry of toxic substances and microorganisms. Natural ingredients, phytonutrients, microbial metabolites, dairy-derived actives, minerals, and animal protein components have long been believed to benefit healthy skin aging. Indeed, from a sustainable point of view, this new application could provide in the near future a method of recycling for food companies, developing cost-efficient processing methods, decreasing the negative impacts of wastes on the environment, and providing other economic advantages for companies.
Coffee is one of the most popular beverages of the world and the second most traded commodity.4 Usually, each coffee fruit contains two coffee beans, each one covered by a thin, tight skin called silverskin.5 Coffee silverskin (Figure 1) is obtained after roasting coffee beans; it is completely removed during coffee beverage production. This by-product has no commercial value, being normally discarded as a solid waste. Recent advances in industrial biotechnology lead to potential opportunities for economic valorization of this by-product.6 Different authors have intensively studied this coffee by-product. Regarding its macronutritional composition, is well documented to contain high amounts of dietary fiber (50–60%, including 85% soluble fiber and 15% insoluble fiber), protein (16.2–19%), and minerals (4–5%).7–9 However, the exact mineral composition of coffee silverskin has not been clarified.10 The high antioxidant capacity of coffee silverskin, particularly in chlorogenic acid, probably owing to the concentration of phenolic compounds, has also been detailed.7,11–14 Also, the caffeine content is high and similar to that of coffee beans.11–12 These compounds are believed to provide in vivo protection against free radical damage. As with coffee beans, coffee silverskin contains several classes of health compounds such as phenolics, diterpenes, xanthines, and vitamin precursors.15 However, few studies reported the use of by-products as antioxidant sources for the cosmetic field.3,13,16–19 According to these research studies, the majority of these antioxidants are polyphenols and isoflavones. The question is, how can we validate coffee silverskin as a new cosmetic ingredient, particularly as an anti-wrinkle ingredient?
Recent changes in regulatory requirements and social views on animal testing have prompted the development of reliable alternative tests for predicting skin and ocular irritation potential of products based on new raw materials. Nevertheless, to guarantee the safety of these new ingredients, stability and toxicity assays should be performed to avoid the presence of irritating constituents. Rodrigues et al. evaluated the cytotoxicity of coffee silverskin in monolayers of different skin cell lines.13 No cytotoxicity was observed. Also, a number of in vitro three-dimensional models have been developed to assess potential skin or eye irritants by the cosmetics industry, such as the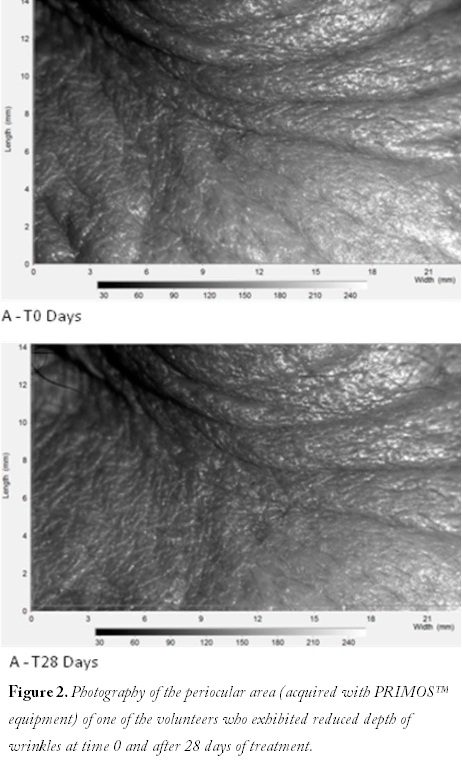
reconstructed human epidermis test (EpiSkin™) and the human corneal epithelial model (SkinEthic™ HCE), respectively. The same research group performed these assays in three different extracts of coffee silverskin. The histology of the models after extract application was also analyzed. The in vitro results demonstrated that extracts were not classified as irritants, and the histological analyses proved that extracts did not affect either model’s structure. The contents of caffeine, 5-hydroxymethyl furfural, and chlorogenic acid were quantified after the epidermal assay, proving that caffeine was in a similar amount to coffee beans. Thus, this new extract could clearly be used as an anticellulitis agent. The in vivo test carried out with the most promising extract (hydroalcoholic) showed that, with respect to irritant effects, it can be regarded as safe for topical application, because no skin irritation was observed. Rodrigues et al. also incorporated coffee silverskin into different cosmetic formulations, such as a body cream and a hand cream.19–20 In vitro results were completely satisfactory, as no toxicity was observed in keratinocytes nor in fibroblasts. Also, the in vivo assays demonstrated that both formulations were safe. The sensorial analyses performed with volunteers demonstrated complete satisfaction with both products. Recently (data not available) a facial formulation with coffee silverskin (formulation A) and the same cream enriched with hyaluronic acid (formulation B) were compared in vivo. Formulations were applied twice a day by volunteers (n = 20 for each formulation) during 28 days. The influence on skin hydration and viscoelastic properties was investigated with validated devices (Corneometer® and Cutometer® ). Wrinkle depth, roughness, volume of cavities, and Visioface® images were analyzed at time 0 and after 28 days. Volunteers were asked about efficacy perception. Results revealed that permeation of coffee silverskin extract in pig ear skin after 8 h was about 20%. No cytotoxicity was observed for both formulations. Significant changes in skin hydration and viscoelastic parameters were detected for both formulations, without differences between formulations. However, no differences were observed regarding wrinkle depth, roughness, and volume of cavities for both formulations (Figure 2). Coffee silverskin represents an effective ingredient for cosmetic creams that are intended to increase skin hydration and firmness, with no in vivo differences regarding hyaluronic acid.
In the same context, the potential for nanostructured lipid carriers loaded with caffeine, obtained from coffee silverskin, as a new approach for topical therapy of cellulitis, has been investigated in our group with interesting results (data not available).
Acknowledgements
The authors declare that there are no conflicts of interest. Francisca Rodrigues is grateful to the Foundation for the Science and Technology (Portugal) for the Ph.D. grants SFRH/BDE/51385/2011, financed by POPH-QREN and subsidized by the European Science Foundation.
1. Behrens, A, Giljum, S, Kovanda, J, Niza, S. The material basis of the global economy: Worldwide patterns of natural resource extraction and their implications for sustainable resource use policies. Ecol. Econ. 64(2):444-453 (2007).
2. McPhee, D, Jain, R. Price, performance, supply and sustainability all-in-one—The Amyris case. H&PC Today 10(5):71-72 (2015).
3. Rodrigues, F, Palmeira-de-Oliveira, A, das Neves, J, Sarmento, B, Amaral, MH, Oliveira, MB. Medicago spp. extracts as promising ingredients for skin care products. Ind. Crop. Prod. 49:634-644 (2013).
4. Murthy, PS, Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recy. 66:45-58 (2012).
5. Saenger, M, Hartge, EU, Werther, J, Ogada, T, Siagi, Z. Combustion of coffee husks. Renew. Energ. 23(1):103-121 (2001).
6. Pandey, A, Soccol, CR, Nigam, P, Brand, D, Mohan, R, Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 6(2):153-162 (2000).
7. Borrelli, RC, Esposito, F, Napolitano, A, Ritieni, A, Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 52(5):1338-1343 (2004)
8. Pourfarzad, A, Mahdavian-Mehr, H, Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT–Food Sci. Technol. 50(2):599-606 (2013).
9. Napolitano, A, Fogliano, V, Tafuri, A, Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 55(25):10499-10504 (2007).
10. Narita, Y, Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 61:16-22 (2014).
11. Bresciani, L, Calani, L, Bruni, R, Brighenti, F, Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 61:196-201 (2014).
12. Narita, Y, Inouye, K. High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 135(3):943-949 (2012).
13. Rodrigues, F, Palmeira-de-Oliveira, A, Das Neves, J, Sarmento, B, Amaral, MH, Oliveira, MB. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 53(3):386-394 (2015).
14. Sato, Y, Itagaki, S, Kurokawa, T, Ogura, J, Kobayashi, M, Hirano, T, Sugawara, M, Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 403:136-138 (2011).
15. Alves, RC, Casal, S, Alves, MR, Oliveira, MB. Discrimination between arabica and robusta coffee species on the basis of their tocopherol profiles. Food Chem. 114(1):295-299 (2009).
16. Rodrigues, F, Almeida, I, Sarmento, B, Amaral, MH, Oliveira, MB. Study of the isoflavone content of different extracts of Medicago spp. as potential active ingredient. Ind. Crop. Prod. 57:110-115 (2014).
17. Rodrigues, F, Gaspar, C, Palmeira-de-Oliveira, A, Sarmento, B, Helena Amaral, M, Oliveira, MB. Application of coffee silverskin in cosmetic formulations: Physical/antioxidant stability studies and cytotoxicity effects. Drug Dev. Ind. Phar. 22:1-8 (2015).
18. Rodrigues, F, Pereira, C, Pimentel, FB, Alves, RC, Ferreira, M, Sarmento, B, Amaral, MH, Oliveira, MBPP. Are coffee silverskin extracts safe for topical use? An in vitro and in vivo approach. Ind. Crop. Prod. 63:167-174 (2015).
19. Rodrigues, F, Sarmento, B, Amaral, MH, Oliveira, MB. Exploring the antioxidant potentiality of two food by-products into a topical cream: Stability, in vitro and in vivo evaluation. Drug Dev. Ind. Phar., doi: 10.3109/03639045.2015.1088865 (in press).
20. Rodrigues, F, Gaspar, C, Palmeira-de-Oliveira, A, Sarmento, B, Helena Amaral, M, Oliveira MB. Application of coffee silverskin in cosmetic formulations: Physical/antioxidant stability studies and cytotoxicity effects. Drug Dev. Ind. Phar. 42(1):99-106 (2016). n

