Wildlife Drug Delivery: The Case of Avian Malaria in a New Zealand Penguin
Authors: Stacey Woodsa, Natalie J. Medlicotta, Lisa Argillab and Arlene McDowella
a School of Pharmacy, University of Otago, Dunedin, New Zealand
b The Dunedin Wildlife Hospital, Dunedin, New Zealand
Wildlife medicine is a specialised section of veterinary medicine where the patients are morphologically very diverse and from a range of different ecological environments. With the numerous threats of extinction to wildlife globally, the ability to effectively treat this group of animals is becoming increasingly more critical. The COVID-19 pandemic has also made us acutely aware of the important link between human health and wildlife health.
New Zealand’s bird fauna
New Zealand is an isolated land mass where the wildlife has evolved in isolation and so the native New Zealand fauna is unique. There are no native land mammals in New Zealand and so the native fauna is comprised solely of birds and reptiles. An iconic bird species in New Zealand is the endangered yellow-eyed penguin (Megadyptes antipodes) that only occurs in southern New Zealand (Fig. 1).
The Wildlife Hospital is a recently-established veterinary facility in Dunedin, New Zealand that specialises exclusively in the treatment of native New Zealand species, with the majority of the patients being birds. The Wildlife Hospital treats sick and injured animals with the aim of rehabilitation for subsequent release back to their native habitat. Treatment of birds often involves surgery with the associated administration of anti-infective agents, anaesthetics and analgesic drugs. The endemic yellow-eyed penguin is the species with the highest number of admissions to the Wildlife Hospital.
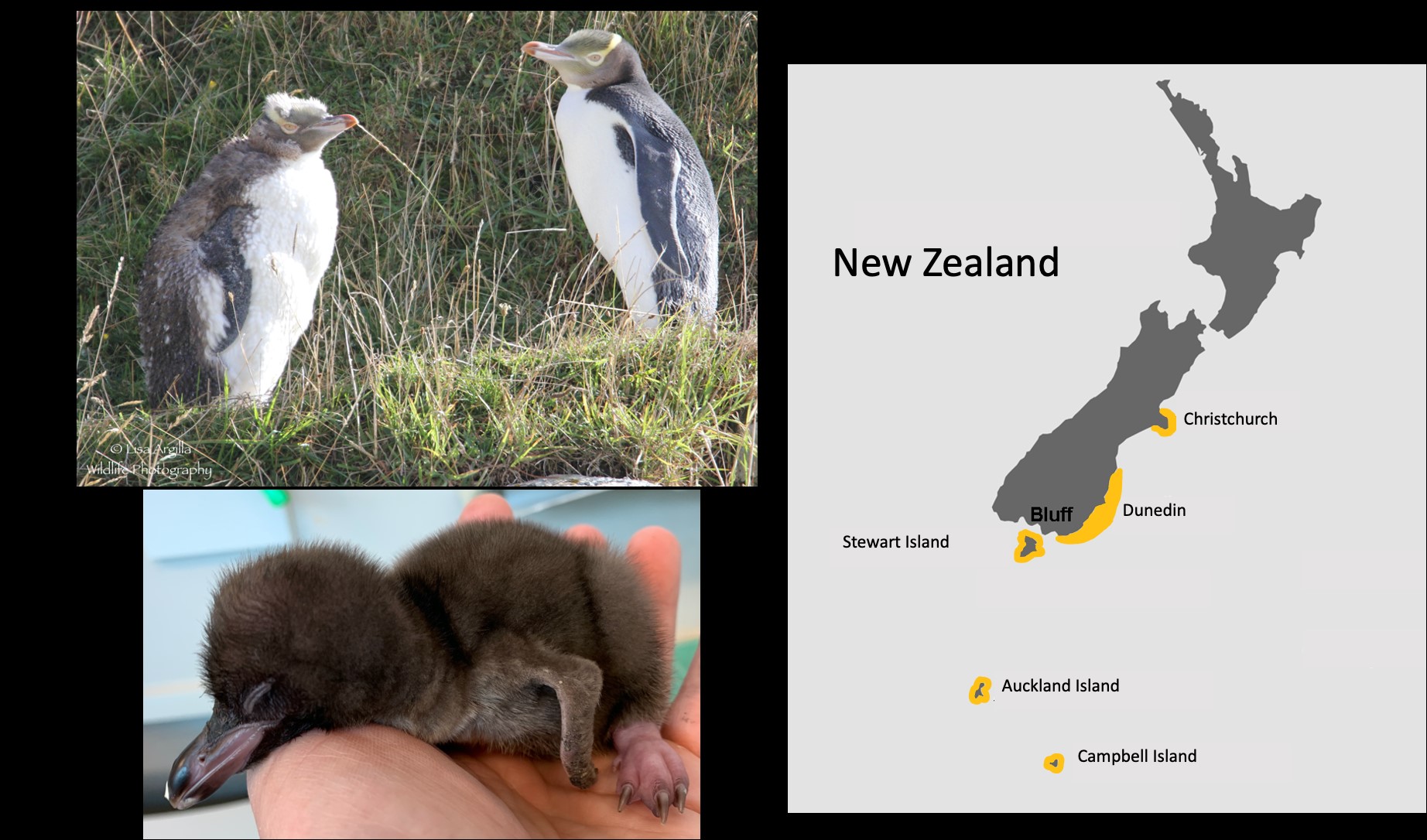
Figure 1. (a) Photo of a juvenile (left) and adult (right) yellow-eyed penguin photographed in the field in New Zealand. Note the distinctive band of yellow feathers around the eye that give this bird its common name. The Māori name for the yellow-eyed penguin is hoiho. Photos by L. Argilla. (b) A hoiho chick. (c) Distribution map of the yellow-eyed penguin (shown in yellow) on the south-east coast of Southern New Zealand and the subantarctic islands.
The diseases that occur in New Zealand birds mirror that in bird species from other parts of the world and also other animals and includes parasitic infections, viruses and bacterial infections. In New Zealand, avian malaria is a disease that is a significant threat to the survival of the endangered, endemic yellow-eyed penguin (Sturrock and Tompkins 2008). This disease is transmitted by mosquitoes and prior to 2018 has only been diagnosed on very rare occasions in yellow-eyed penguins. Due to the warming climate, mosquito numbers are increasing further to the south and with them, the incidence of avian malaria and associated mortality. Yellow-eyed penguins have not evolved to cope with this disease and as such have no immunity to avian malaria.
Chloroquine is the preferred drug of choice for malaria prevention, however as a Schedule 29 drug, it is not available for veterinary use in New Zealand. The alternative antimalarial product malarone has been observed to be effective in preventing malaria in humans, however very little use has occurred in animals. Malarone has been used prophylactically in yellow-eyed penguins with 100% protection observed in this species in rehabilitation compared to untreated birds over three seasons in Dunedin.
What’s the dose?
Drug action and effect depends upon pharmacokinetics. Blood drug levels in turn are influenced by the physiological processes that underlie the absorption, distribution, metabolism and elimination of drugs (Toutain et al. 2010).
Species-specific information on physiology relevant to appropriate drug dose is currently lacking for a range of wildlife. As pharmacokinetic studies that document the time course of drug in the blood of wild animals are very rare, empirical rather than evidence-based prescribing is common. Critically, there is a paucity of information on dosing regimens for birds and this can lead to sub-therapeutic treatment (Hunter et al. 2008). In the case of penguins, we have identified fewer than 10 pharmacokinetic studies in only three species (African, Magellanic and Humbolt penguins) for the drugs enrofloxacin, terbinafine, itraconazole and voriconazole and the analgesics tramadaol and meloxicam. A notable feature of the data from these studies is that penguins are distinct from other species of bird in their pharmacokinetics (Fig. 2).
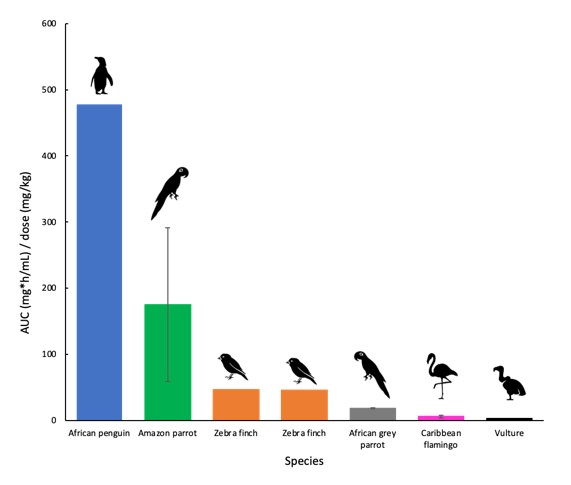
Figure 2. AUC adjusted for dose of meloxicam administered IM to a range of bird species (Woods et al. unpublished).
Current practices for administration of medicines to New Zealand native birds involves the extrapolation of data from other species. In the case of malarone for the treatment of avian malaria, the dose given to yellow-eyed penguins is based on scaling down of the human dose. The pharmacokinetics of malarone is understood for humans, however it has not been documented in any bird species, including the yellow-eyed penguin. With the significant differences in anatomy, physiology and life histories between birds and mammals, extrapolation of data from one species to another has inherent errors. Consequently, there is a critical need for robust pharmacokinetic data from the target species.
Determining the pharmacokinetics of the anti-malarial product malarone is not straight forward because malarone is a combination tablet product that contains atovaquone 250 mg and proguanil hydrochloride 100 mg. It is also anticipated that each drug will have different pharmacokinetic profiles, which necessitates different analytical assays to be developed that can be applied for the quantification of malarone in penguin plasma.
Delivery systems
The majority of pharmaceutical products do not have an avian equivalent and so adjustment to formulations designed for other species is required. For example, for the parenteral route of administration, the relatively small body size and small muscle mass in some birds compared to mammals can create difficulties using this route of administration.
The oral route is the most commonly utilized for administration of medicines to birds (typically liquids), often either in-feed or in-water. The differences in the diet of birds compared to mammals also leads to unique specializations in gastrointestinal tract anatomy (e.g. the presence of a crop and gizzard) that can influence drug absorption and so onset of drug action. In addition, for some species of bird, including penguins, incorporating the active drug in fish for consumption is adopted as an acceptable delivery strategy. Utilizing this type of “delivery system” will also require an understanding of how this meal can influence drug absorption and so bioavailability. Using the case study of malarone in the yellow-eyed penguin, the atovaquone component of this medicine is very lipophilic and may require lipid digestion products to be produced in order for absorption to occur. Such specialised requirements also emphasises that translation to other bird species is not trivial and that species-specific information is key.
In the wildlife setting, remote delivery is a prudent strategy that means the animals do not need to be handled. In these situations where contact with the patient is challenging, utilizing modified-release strategies that can reduce the frequency of application would also be a significant advantage in the treatment of birds, as it is for other wildlife. Drug delivery technologies used in human and veterinary medicine (Carvalho et al. 2021) can be applied in the wildlife setting, with appropriate modifications tailored to the target species (McDowell et al. 2006). This is a particularly fertile area of research as there are currently many gaps in our knowledge to design appropriate formulations for wildlife applications.
Conclusions
Diseases such as avian malaria have potential to result in mass mortality and localized extinction of avian populations. Data to guide the correct drug dose is essential for effective treatment of wildlife. Understanding species-specific requirements for drug delivery can contribute to achieving conservation goals and improved health of wildlife. Improving awareness of drug delivery opportunities through collaboration of wildlife researchers and pharmaceutical scientists can help to uncover valuable new approaches to improve drug therapy for wildlife.
References
Carvalho SG, Silvestre ALP, dos Santos AM, Fonseca-Santos B, Rodrigues WD, Gremião MPD, Chorilli M and Villanova JCO (2021). Polymeric-based drug delivery systems for veterinary use: State of the art. International Journal of Pharmaceutics 604: 120756
Hunter RP, Mahmood, I and Martinez MN. Prediction of xenobiotic clearance in avian species using mammalian or avian data: how accurate is the prediction? Journal Veterinary Pharmacology and Therapeutics 2008;31: 281-284
McDowell A, McLeod BJ, Rades T and Tucker IG (2006). Application of pharmaceutical drug delivery for biological control of the common brushtail possum in New Zealand. Wildlife Research 33: 697-689
Sturrock HJW and Tompkins DM (2008). Avian malaria parasites (Plasmodium spp.) in Dunedin and on the Otago Peninsula, southern New Zealand. New Zealand Journal of Ecology 32: 98-102
Toutain P-L, Ferran A and Bousquet-Mélou A. Species differences in pharmacokinetics and pharmacodynamics. In Comparative and Veterinary Pharmacology (Eds E. Cunningham, J. Elliot and P. Lees). Springer, Heidelberg, 2010

