Introduction
The emergence of antibiotic resistant bacteria presents a global threat to public health, with the death toll resulting from methicillin-resistant Staphylococcus aureus (MRSA) having already overtaken that of HIV/AIDS and tuberculosis combined.1-2 As such, the search for new antimicrobial therapies is more important than ever, and antimicrobial agents with a reduced prospect of inducing resistance in target organisms are of particular interest. One particularly attractive alternative to conventional small molecule antibiotics are antimicrobial peptides (AMPs).
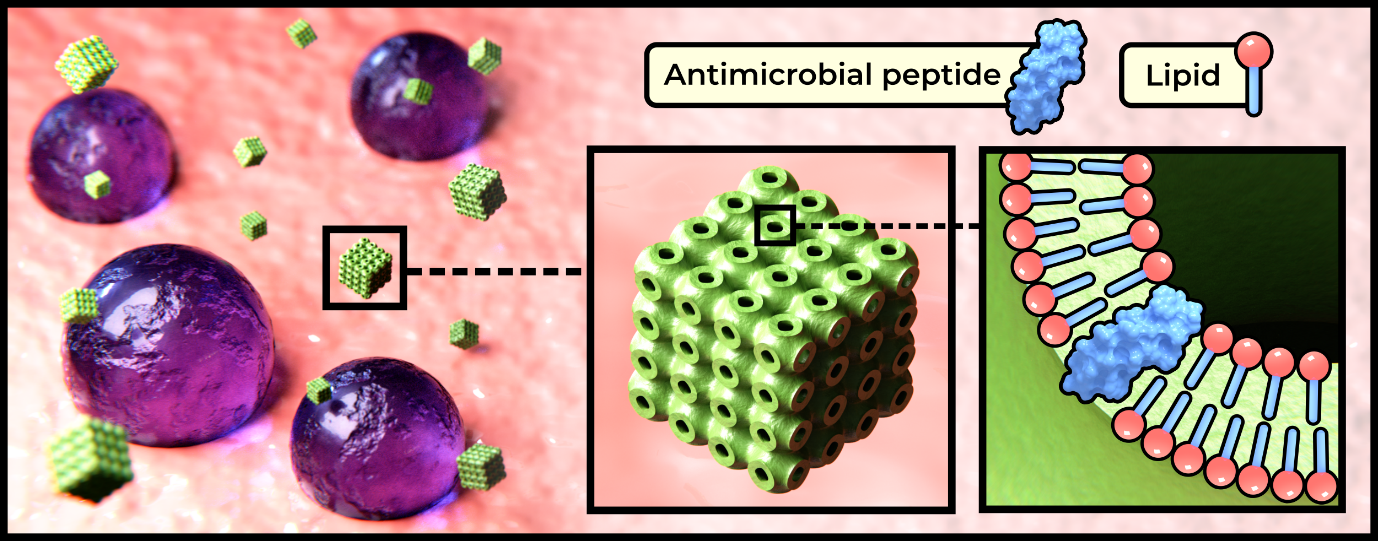
The efficacy of these short chain, often cationic biomolecules is, however, limited by their chemical instability under biological conditions, and potential toxicity resulting from poor selectivity towards target organisms. With an aim to address these issues, recent work from within our research group has focussed on the encapsulation of AMPs within lipidic inverse bicontinuous cubic phase nanoparticles (cubosomes).3 These nanoparticles, which consist of a single lipid bilayer approximating the geometry of a triply periodic minimal surface (illustrated in Fig 1), offer a biomimetic environment for peptide encapsulation, high surface area to volume ratio, and potential for further functionalization enabling the development of targeted delivery and release mechanisms. We present herein a combination of data from our previous research,3 as well as unpublished data.
Experimental Work:
AMP encapsulating cubosomes were prepared via the co-dissolution of peptides and lipid in trifluoroethanol, followed by solvent removal in vacou resulting in a dry lipid mixture, which was dispersed in a solution of steric stabilizer (F127) using ultrasonication. Three different AMPs were investigated in this work; gramicidin A’, alamethicin, and melittin. Each peptide was seperately encapsulated in cubosomes comprised of monoolein or the branched chain lipid phytantriol.
To ensure retention of the nanoparticle structure upon peptide loading, synchrotron radiation small angle X-ray scattering (SR-SAXS) was employed to probe changes in the mesoscale structure of the cubosomes with increasing AMP concentration. The measured phase and lattice parameter (Å) enabled the determination of appropriate loading concentrations for each AMP in both monoolein and phytantriol based cubosomes. Based on this data, peptide concentrations in which the cubic phase is retained were selected for further investigation.
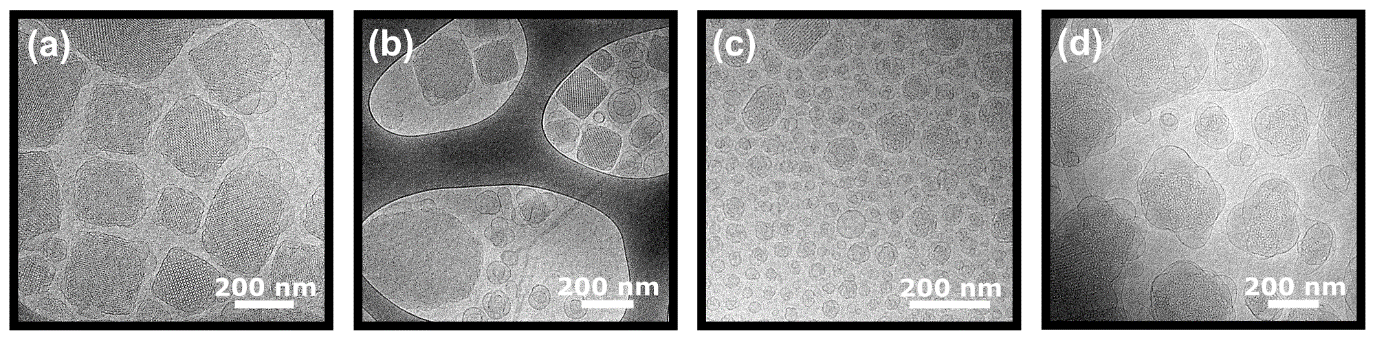
Select monoolein (shown in Fig. 3) and phytantriol cubosome formulations were subjected to cryogenic transmission electron microscopy, to provide further elucidation of the cubosome structure. In each case, retention of the cubic structure was confirmed, and additional morphological observations could be made. In monoolein + 2 mol% gramicidin A’ samples, vesicles, disordered sponge-phase particles, and peptide precipitate were observed in addition to well-ordered cubosomes. In the monoolein + 0.5 mol% melittin samples, and to a lesser extent monoolein + 2 mol% alamethicin samples, an increase in the number of small unilamellar and multilamellar vesicles was observed, correlated with a decrease in z-average measured via dynamic light scattering (data not shown).
Circular dichroism was employed in order to probe the membrane association and resulting secondary structure of AMPs encapsulated in different cubosome formulations. This is possible because when unencapsulated, the hydrophobic peptides gramicidin A’ and alamethicin precipitate out of solution, thus contributing negligibly to the CD signal. Similarly, for the water-soluble melittin, there is a significant difference in CD spectra between freely solubilized peptide and that which is associated with the membrane. Thus, in each case the magnitude and characteristic shape of the CD signal can be used to qualitatively gauge the level of peptide loading in each cubosome formulation.
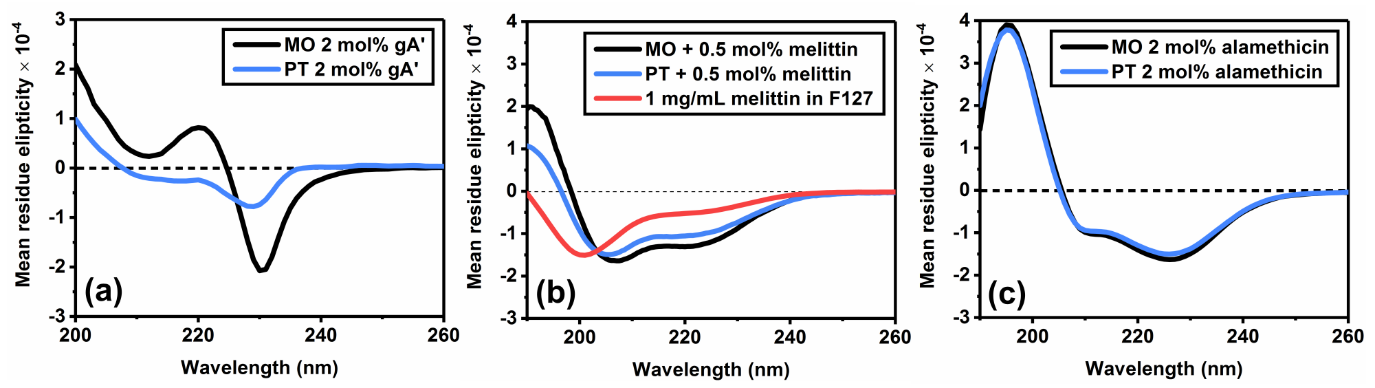
The CD spectra of 2 mol% gramicidin A’ in cubic phase bilayers indicates that the peptide is present as a combination of two different conformations,4 the proportions of which are influenced by lipid composition. Importantly, significant reductions in the magnitude of the CD signal in phytantriol samples indicate reduced encapsulation of gramicidin A’.
In 0.5 mol% melittin formulations, a significant shift from an extended conformation (freely solubilized) to an α-helix (membrane associated) was seen in both monoolein and phytantriol samples. In monoolein cubosomes, the increased magnitude of the CD signal is indicative of greater loading efficiency. In 2 mol% alamethicin formulations, the respective CD spectra for monoolein and phytantriol based cubosomes are near identical, strongly indicative of a similar level of peptide loading efficiency.
Upcoming work
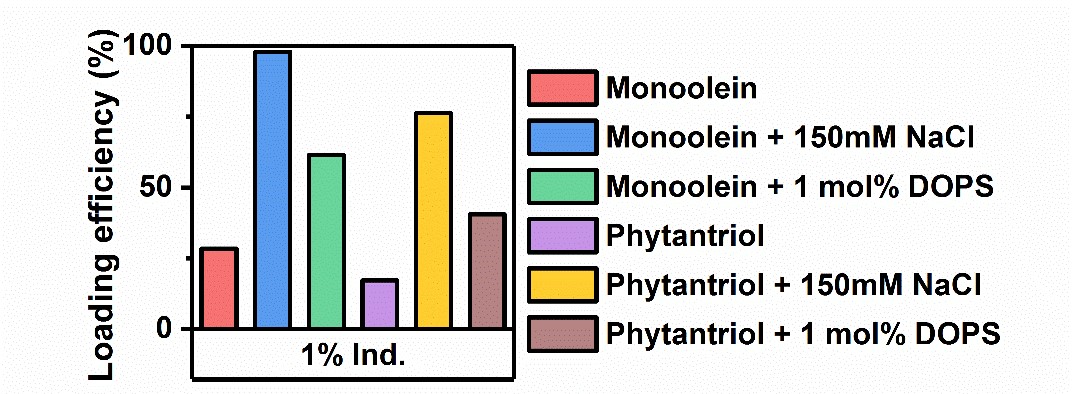
We herein present select data from a forthcoming publication, in which our group has expanded this work to include several additional AMPs, encapsulated in a variety of cubosome formulations. Using centrifugal filtration to separate cubosomes from the surrounding solution, followed by UV-Vis spectroscopy, we were able to quantitatively determine peptide loading efficiency (%). In Fig. 4 we present the loading efficiency data for the cationic AMP indolicidin. For this peptide, loading efficiency in pure monoolein and phytantriol cubosomes was initially low, however, through the inclusion of 1 mol% of the anionic phospholipid DOPS in the cubosome formulation, we were able to significantly increase peptide loading due to electrostatic interactions between the peptide and the membrane. Loading could be further increased by the use of a high ionic strength buffer (150 mM NaCl). These findings were further confirmed through the use of CD spectroscopy (data not shown).
In this work, cubosome formulations which demonstrated peptide loading efficiency >50% were carried through to antimicrobial assays, using a broth microdilution assay to determine minimum inhibitory concentrations (MIC) against Staphylococcus aureus (ATCC 25923).
|
Table 1: The determined MIC values of indolicidin, as free peptide and encapsulated in three different cubosome formulations, when tested against S. aureus.
The MIC of unencapsulated indolicidin was determined to be 32 ug/mL, in keeping with literature values. Encapsulation in phytantriol (150 mM NaCl) saw the MIC decrease by two dilutions, indicating that activity was enhanced by this particular formulation. Interestingly, encapsulation in equivalent monoolein cubosome formulations lead to a decrease in activity, an effect currently being explored.
Discussion
SR-SAXS and cryo-TEM proved particularly useful in determining cubosome morphology subsequent to peptide loading. The results of these experiments demonstrate that AMPs can be successfully loaded into cubosomes of various lipid compositions at biologically useful concentrations, without loss of the cubosome structure. The application of CD spectroscopy allows for analysis of the peptides secondary structure, and qualitative determination of peptide loading efficiency, which could be quantified using filtration and UV-Vis techniques. Of particular note, we find that peptide loading efficiency (%) can be significantly increased via manipulation of electrostatic charge.
The finding that encapsulation within cubosomes increases the antimicrobial activity of select peptides is also of interest, and in future work we plan to examine the mechanisms involved in cubosome uptake into bacteria, including the influence of lipid composition and surface charge. We anticipate that this work will enable the design of effective, targeted delivery vehicles for antimicrobial peptides, and perhaps see the emergence of a new class of antimicrobial therapeutics.
References
1. Boucher, H. W.; Corey, G. R., Epidemiology of methicillin-resistant Staphylococcus aureus. Clinical infectious diseases 2008, 46 (Supplement 5), S344-S349.
2. Klevens, R. M.; Edwards, J. R.; Tenover, F. C.; McDonald, L. C.; Horan, T.; Gaynes, R.; System, N. N. I. S., Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clinical infectious diseases 2006, 42 (3), 389-391.
3. Meikle, T. G.; Zabara, A.; Waddington, L.; Separovic, F.; Drummond, C.; Conn, C. E., Incorporation of antimicrobial peptides in nanostructured lipid membrane mimetic bilayer cubosomes. Colloids and Surfaces B: Biointerfaces 2017, (152), 143-151.
4. Meikle, T. G.; Conn, C. E.; Separovic, F.; Drummond, C. J., Exploring the structural relationship between encapsulated antimicrobial peptides and the bilayer membrane mimetic lipidic cubic phase: studies with gramicidin A'. RSC Advances 2016, 6 (73), 68685-68694.

