Targeted penetration of active ingredients into the skin using nanotransport systems - challenges and research findings
Introduction
The barrier of the stratum corneum (SC) represents the biggest challenge for the transport of drugs into the viable skin. Drug delivery through the skin is an essential pathway; in this case, the drugs are topically applied, thus reducing side effects that may occur with systemic administration. However, the skin has an excellent barrier that makes the uptake of polar and large molecules very difficult or even impossible [1, 2]. Only 0.1 to 1% can pass this barrier if the molecular mass is below 500 Dalton [3]. In this review, sophisticated methods and their application to study the penetration efficacy of active ingredients into the skin are presented. Microscopic and spectroscopic methods are demonstrated, and also the extent to which their application provides an overall picture of the assessment of drug penetration. Nanotransport systems loaded with the drug of interest are in the focus of the presented research approaches.
Penetration pathways
Several skin penetration pathways for topically applied substances are known (Figure 1): Diffusion through the intercellular space of the SC, the transcellular route involving the corneocytes and penetration along the hair follicles (HFs).
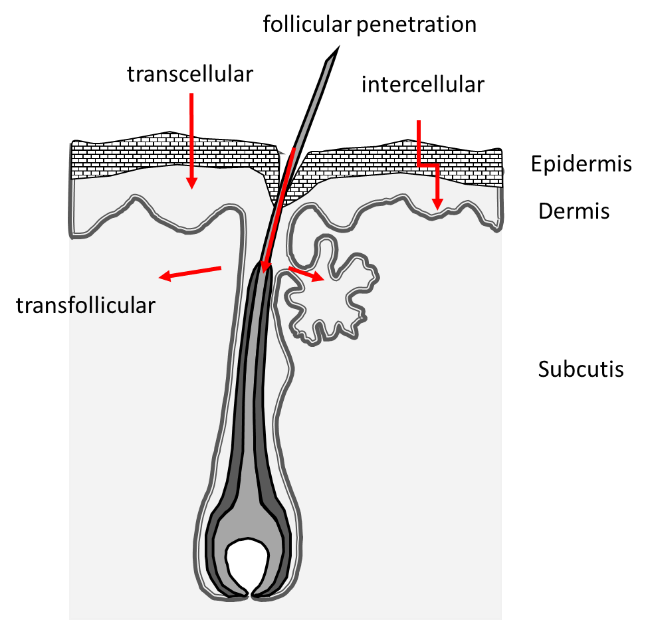
For many substances, it is assumed that two or even all penetration routes are used in parallel. In most cases, topically applied drugs penetrate into the upper corneocyte layers of the SC, which are subject to rapid desquamation and thereby depletion of the drug reservoir. HFs have an essential role as a penetration route to overcome the skin barrier [4, 5]. Studies have shown that skin areas with available follicular pathway can deliver the model drug caffeine faster and in a higher amount than a comparable area where this pathway is blocked [6, 7]. The targeted follicle closure provides information about the penetration path of active ingredients into the skin, whereby the varnish to be used for a follicle closure as well as the application technique must be adapted to the substance to be examined [8].
Strategies to overcome the skin barrier
Barrier disruption
To bring substances into the skin, mechanical disruption of the SC is possible using tape stripping, microneedles or laser perforation [9-11]. By means of two-photon tomography (2PT) and the use of adhesive tape strips and cyanoacrylate stripping (CS), a comparative characterisation with regard to the efficiency of these two methods for the targeted induction of a barrier disruption in excised skin (human/porcine) was investigated. After performing of 30 tape strips or 2 times CS, approximately 5 µm of the SC remain on human skin, whereas 50 tape strips or 4 times CS remove the complete SC. In porcine skin, 2-3 µm of the SC still remained. Improved removal of the SC is accompanied by an increased permeability coefficient (as shown with the hydrophilic nitroxide spin probe 3-(Carboxy)-2,2,5,5-tetramethyl-1-pyrrolidinyloxy (PCA)). To simulate an ex vivo barrier disorder, 30 tape strips or 3 CS are recommended [12].
Penetration enhancer
Penetration enhancers, including chemicals such as DMSO or ethanol [13] disturb the SC, which is not recommendable for the treatment of skin diseases with barrier deficiencies. Ethanol alone should not be used for penetration enhancement, but in combination with water, it can enhance the penetration of hydrophilic substances as shown for the hydrophilic substance PCA [14].
Drug delivery systems
One way to improve the transport of active ingredients is the use of nanotransport systems (NTS). The advantages of such NTS are higher transport concentrations of active ingredients to target structures, improved solubility of highly hydrophobic active ingredients, increased chemical and physical stability of drugs, and that a drug release can be controlled. It is crucial that the active ingredient is released at the target structure and, in addition to rapid release and effect ideally a sustained release should also be achieved, which allows a reduction in the application rate. The NTS used must have the highest possible loading. In addition, the solvent or formulation also influences the penetration of substances into the skin [15, 16]. For most nanocarriers (NCs) the mechanisms of active penetration enhancement are not yet clear. Nevertheless, NCs have been shown to likewise enhance topical drug delivery processes into the skin [17-20] and also the oral mucosa [21]. HFs display a long term reservoir for NCs, and only hair growth, sebum production or degradation of the NCs will result in a depletion of the reservoir [22]. The transfollicular absorption of small molecules diffusing from the NCs into the blood vessels which surround the HFs has also been reported as a possible penetration pathway [23]. NCs can be specifically designed to have a significant effect on the penetration of the amount of drug, its release and the site of action. Substances for the treatment of skin diseases require penetration through the SC, whereby a barrier disorder can often facilitate access in the acute inflammatory phase.
Methods to monitor penetration
The penetration of substances or NCs can be investigated using several in vitro methods on membranes or reconstructed human skin, ex vivo on excised human or porcine skin and in vivo on human volunteers, if all ingredients are approved to be applicable. Conventional methods such as tape stripping or Franz cells are available but also optical, and biophysical sophisticated techniques can be applied (Table 1). If drugs or NCs are labelled, or fluorescent substances replace the drugs, laser-scanning microscopy (LSM) is frequently used to monitor the distribution of the drug within the skin and HFs [18, 24, 25]. Confocal laser scanning microscopy (CLSM) is used to determine the follicular penetration depths. For CLSM the substances of interest must be fluorescent [20, 26]. Spin- labelled drugs can be used to monitor the release of drug from a carrier in vitro / ex vivo by electron paramagnetic resonance (EPR) spectroscopy. Furthermore, the EPR technique provides non-destructive quantitative data of the drug in the skin [17-19, 27, 28]. The EPR spectroscopy is a well-developed method for the determination of environmental properties of an EPR-labeled substance. This method provides information about the microenvironment and thus about the localisation of a drug within a carrier system or a NC. It provides dynamic and structural information about a carrier system and lends itself excellently for the observation of drug release processes. For the structure elucidation, a multi-frequency analysis is essential from low (L-Band, 1-3 GHz) to high frequency (W-band, 94 GHz) which supplies different information about the sample to be analysed, which in sum gives an overall picture [29]. Semi-quantification is also possible using micro-dialysis. It simulates the real condition much better than the Franz cell experiments [8, 30]. For substances providing auto-fluorescence, fluorescence lifetime imaging (FLIM) can be applied to track the substances with high special resolution [31]. Another label-free technique is the confocal Raman microspectroscopy, which allows the molecule-specific representation of different skin components providing penetration profiles of drugs, carrier and formulations [31, 32]. Two-photon tomography can be used to examine topically applied substances in the skin in vivo [12].
Table 1: Methods to investigate penetration of substances
| Methods | Application | Target |
|---|---|---|
| LSM |
In vivo: 150 µm penetration visualisation Ex vivo: penetration depth into the hair follicles and skin |
Fluorescent dye, high sensitivity |
| 2PT | Visualisation of drugs with high resolution, chemical information by FLIM | Fluorescent substance or auto-fluorescent component |
| Raman | In vivo: Penetration profile | Raman active substances or vesicle |
| EPR |
Drug release, loading in NC, microenvironment, quantification of drug in epidermis, dermis |
EPR label, or spin maker |
| Micro-dialysis | Trans epidermal and trans follicular penetration | Depends on classical analytics, and membrane |
| Differential stripping | Quantification of drug in SC and hair follicle | Chemical drug analysis after extraction |
| Franz cell | Quantification of drugs transdermal | Depends on classical analytics |
Selected research findings with the model drug Dexamethasone
Non triggered drug delivery
The influence of the skin barrier on the penetration behaviour on drugs, and to what extent the skin barrier can be overcome by carrier systems, was investigated by different spectroscopic and microscopic methods using the model drug Dexamethasone (Dx). Dx represents a synthetic glucocorsteroid and is frequently applied for the treatment of skin diseases with an inflammatory character [33, 34]. To improve the penetration of this model drug into the skin, polymer-based carrier systems such as dendritic core-multishell (CMS) nanotransport systems [27], lipid-based particles (nanostructured lipid carriers (NLC) [20, 28], pH triggered (Eudragit®) NCs [17] and nanocrystals [19] were examined. To investigate the penetration behaviour of the model drug Dx with and without NCs in the skin, the drug was covalently labelled with the stable EPR marker PCA. An additional application of further spectroscopic and microscopic approaches (with/without specific labelling) provides information about the overall process of penetration of the drug and the carrier system into the skin, whereby an evaluation and improvement can be aimed at as shown in pre-investigations for the spin-labeled 5-doxyl stearic acid (5DSA) model drug [18].
In general, for DxPCA an enhanced penetration could be shown by EPR investigations, furthermore the release from the carrier system into the SC of porcine skin (ex vivo) and consequently the change in the microenvironment by intercalation of Dx into the lipid layers of the SC could be monitored. DxPCA loaded to carrier systems penetrates better and deeper into the skin in contrast to a standard base cream formulation [19, 20] or an aqueous solution [27] where DxPCA remains mostly on the surface. The penetration of DxPCA loaded to nanostructured lipid carriers (NLC) or dissolved in a base cream formulation into the skin could be traced to the viable epidermis for two penetration periods. The polarity and microenvironment changes, which represent drug release events, could be investigated in detail for both vehicles. The penetration of the vehicle could be followed by confocal Raman microscopy (CRM) [20].
In general, the penetration of a drug is influenced by the formulation itself, the penetration time and the skin condition. A disrupted skin barrier [12] as well as an extended penetration time promote the penetration of a drug into the skin and its release from the respective vehicle. This could be shown by using nanocrystals [19]. Nanocrystals represent a further development of NCs for dermal application. 100% drug loading, a large surface area and an increased adhesion and potential for hair follicle targeting represent their advantages [35]. Dx-nanocrystals improved the amount of drug significantly into intact and barrier-disrupted skin versus base cream formulation. A change in the polarity and microenvironment of Dx-PCA in deeper skin layers could be shown [19]. Based on Dx penetration data, time-dependent drug penetration profiles were computed, based on the 1D general diffusion equation. Spatial variations in diffusivity and free energy were analysed enabling a penetration assessment into the skin [19] and HFs [36].
Drug delivery along the hair follicle
EPR investigations do not enable the investigation of the penetration pathway of the investigated NCs. However, in combination with confocal laser scanning microscopy (CLSM) and a fluorescence marker (nile red or curcumin) the importance of the hair follicle as transport route into the skin could be demonstrated (Figure 2) [18, 20]. Due to their morphological properties, HFs enable a mechanically driven transport process of NCs when forces from outside act on the HFs. Hair has a structure of scales, which are arranged in a certain distance to each other, and if the particle size corresponds approximately to the distance between follicle wall and hair and the cuticle cell thickness of the hair (about 500-600 nm), penetration is most effective [25, 37]. Experimental and in silico data also show that movement of the hair in the hair follicle is a prerequisite for directional transport, and radial movement seems to be more effective than an axial movement of the hair [24, 36]. In addition, the massage frequency, with which a formulation is applied to the skin, also has a significant effect on the follicular penetration depth [38]. Targeted closure of the HFs illustrates their importance for the penetration of topically applied substances loaded to NCs, as shown by dermal microdialysis [6-8].
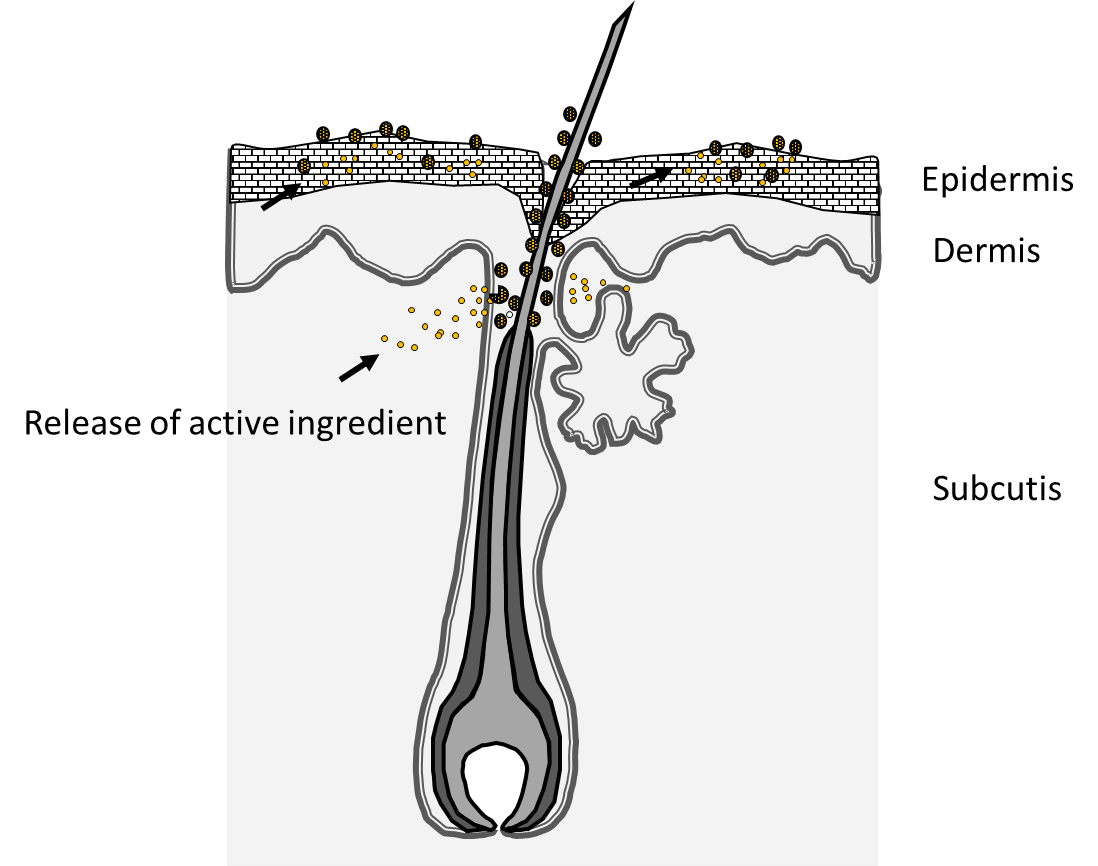
Triggered drug delivery
For the controlled release of drugs into the skin, various parameters can be taken into consideration, as changes in the pH value. Inflamed skin area typically provides higher pH values in comparison to intact skin which has a pH value of around 5.5 in healthy condition [39]. This pH change in inflamed skin can be used as a trigger for a targeted drug release. Using melamine formaldehyde particles, the pH value of freshly plucked hair was determined along the hair shaft down to the hair root by CLSM. A pH decay gradient could be found from the hair root to the tip with mean pH values at the hair root sheath of 6.63±0.09, at the sheath end at 6.33±0.11 and at the hair tip at 6.17± 0.09. The results at the external hair shaft show pH values close to skin pH values, while the hair sheath values were shifted in the direction of the blood pH of ≈7.4. Due to this fact, pH-dependent drug delivery and release inside the hair follicles should occur at pH values 6.5 to 7.0 to ensure the release only inside the follicle [40].
For pH-sensitive nanoparticles (Eudragit®) a high drug load and a controlled drug release and improved penetration of the drug through barrier-disturbed skin was shown [17]. In addition, the NCs were loaded with a fluorescent dye and their penetration into the hair follicles and skin was investigated using LSM. Such carrier systems represent a promising application for skin diseases, which were associated with an increased pH change because they enable a targeted release of the active ingredient on the affected skin lesion where the pH value is increased (> 5.5). Because of the limited volume of the buffer fluid in ex vivo skin, the pH change resulted in a slow swelling of NCs and delayed and sustained the drug release, which could be of clinical significance, given a burst drug release on skin leads to side effects. Thus, a preferential drug release on lesional / barrier-disrupted skin may give the Eudragit® L100 NCs the potential to specifically target diseased skin and reduce side effects on healthy skin [17].
Conclusion
A targeted transport of active ingredients into the skin is influenced by various parameters (Figure 3). The chemical and physical properties of the drug itself must be taken into account, but also the skin area and structure has a great influence on penetration processes. Different techniques can be used to promote the transport of active ingredients into the skin, whereby a distinction between mechanical (e.g. microneedles, tape stripping) chemical enhancers (e.g. DMSO, ethanol) and drug delivery systems can be made. The choice of the technique has a decisive influence on the efficiency of the drug transport into the skin.
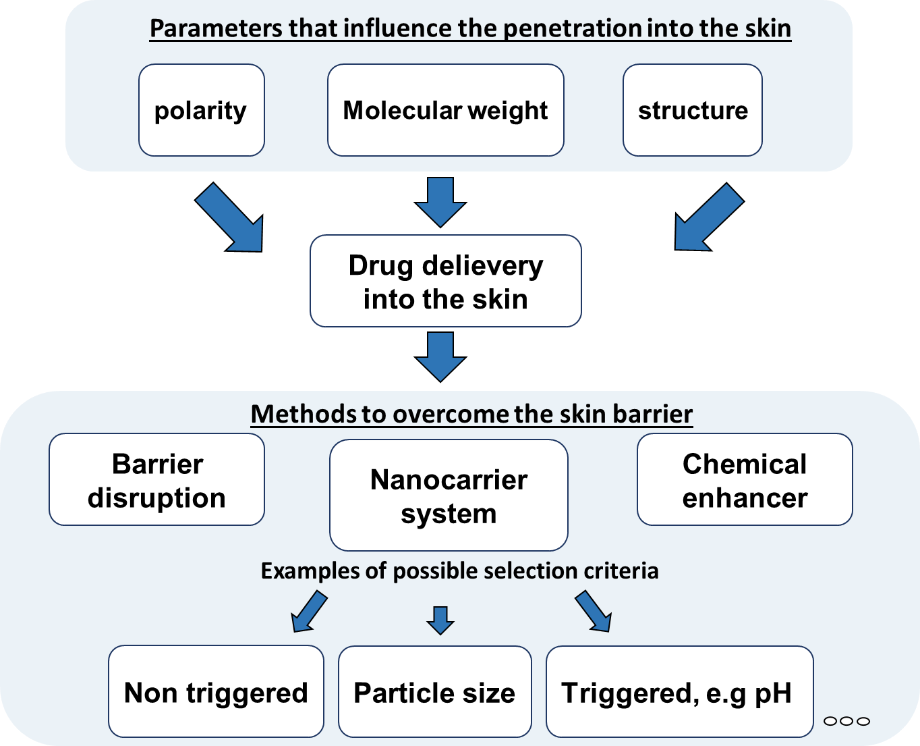
The combination of microscopic and spectroscopic methods with in silico analysis allows to characterise the efficiency of drug transport into the skin and based on the results optimisation can be performed.
References
[1] A.L.M. Ruela, A.G. Perissinato, M.E.D. Lino, P.S. Mudrik, G.R. Pereira, Evaluation of skin absorption of drugs from topical and transdermal formulations, Braz J Pharm Sci, 52 (2016) 527-544.
[2] P.M. Elias, Structure and function of the stratum corneum extracellular matrix, J Invest Dermatol, 132 (2012) 2131-2133.
[3] J.D. Bos, M.M. Meinardi, The 500 Dalton rule for the skin penetration of chemical compounds and drugs, Exp Dermatol, 9 (2000) 165-169.
[4] V. Rodrigues Leite-Silva, M. Mandelli de Almeida, A. Fradin, J.E. Grice, M.S. Roberts, Delivery of drugs applied topically to the skin, Expert Review of Dermatology, 7 (2012) 383-397, DOI: 310.1586/edm.1512.1532.
[5] K.W. Ng, Penetration Enhancement of Topical Formulations, Pharmaceutics, 10 (2018).
[6] N. Otberg, A. Patzelt, U. Rasulev, T. Hagemeister, M. Linscheid, R. Sinkgraven, W. Sterry, J. Lademann, The role of hair follicles in the percutaneous absorption of caffeine, Br J Clin Pharmacol, 65 (2008) 488-492.
[7] N. Otberg, H. Richter, A. Knuttel, H. Schaefer, W. Sterry, J. Lademann, Laser spectroscopic methods for the characterization of open and closed follicles, Laser Phys Lett, 1 (2004) 46-49.
[8] A.L. Klein, M. Lubda, P. Akbarzadeh Taghavi, J. Lademann, I. Beckers, J. von Hagen, H. Kolmar, A. Patzelt, Solvent-Containing Closure Material Can Be Used to Prevent Follicular Penetration of Caffeine and Fluorescein Sodium Salt on Porcine Ear Skin, Skin Pharmacol Physiol, 33 (2020) 117-126.
[9] M. Breternitz, M. Flach, J. Prassler, P. Elsner, J.W. Fluhr, Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping; a randomized, controlled study, Brit J Dermatol, 156 (2007) 231-240.
[10] P.L. Herve, V. Dhelft, C. Plaquet, A. Rousseaux, A. Bouzereau, L. Gaulme, S. Tilleul, M. Ligouis, N. Donne, P.H. Lambert, P. Hong-Thai, W. Wijagkanalan, H.A. Sampson, L. Mondoulet, Epidermal micro-perforation potentiates the efficacy of epicutaneous vaccination, J Control Release, 298 (2019) 12-26.
[11] S.M. Bal, A.C. Kruithof, R. Zwier, E. Dietz, J.A. Bouwstra, J. Lademann, M.C. Meinke, Influence of microneedle shape on the transport of a fluorescent dye into human skin in vivo, Journal of Controlled Release, 147 (2010) 218-224.
[12] P. Dong, V. Nikolaev, M. Kroger, C. Zoschke, M.E. Darvin, C. Witzel, J. Lademann, A. Patzelt, M. Schafer-Korting, M.C. Meinke, Barrier-disrupted skin: Quantitative analysis of tape and cyanoacrylate stripping efficiency by multiphoton tomography, Int J Pharm, 574 (2020) 118843.
[13] T. Haque, M.M.U. Talukder, Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum, Adv Pharm Bull, 8 (2018) 169-179.
[14] P. Dong, C. Teutloff, J. Lademann, A. Patzelt, M. Schafer-Korting, M.C. Meinke, Solvent Effects on Skin Penetration and Spatial Distribution of the Hydrophilic Nitroxide Spin Probe PCA Investigated by EPR, Cell Biochem Biophys, 78 (2020) 127-137.
[15] F. Knorr, A. Patzelt, M.C. Meinke, A. Vogt, U. Blume-Peytavi, E. Ruhl, J. Lademann, Interactions of Nanoparticles with Skin, Nanosci Technol, (2019) 329-339.
[16] A. Vogt, C. Wischke, A.T. Neffe, N. Ma, U. Alexiev, A. Lendlein, Nanocarriers for drug delivery into and through the skin - Do existing technologies match clinical challenges?, Journal of Controlled Release, 242 (2016) 3-15.
[17] P. Dong, F.F. Sahle, S.B. Lohan, S. Saeidpour, S. Albrecht, C. Teutloff, R. Bodmeier, M. Unbehauen, C. Wolff, R. Haag, J. Lademann, A. Patzelt, M. Schafer-Korting, M.C. Meinke, pH-sensitive Eudragit(R) L 100 nanoparticles promote cutaneous penetration and drug release on the skin, J Control Release, 295 (2019) 214-222.
[18] S.B. Lohan, N. Icken, C. Teutloff, S. Saeidpour, R. Bittl, J. Lademann, E. Fleige, R. Haag, S.F. Haag, M.C. Meinke, Investigation of cutaneous penetration properties of stearic acid loaded to dendritic core-multi-shell (CMS) nanocarriers, Int J Pharmaceut, 501 (2016) 271-277.
[19] S.B. Lohan, S. Saeidpour, M. Colombo, S. Staufenbiel, M. Unbehauen, A. Wolde-Kidan, R.R. Netz, R. Bodmeier, R. Haag, C. Teutloff, R. Bittl, M.C. Meinke, Nanocrystals for Improved Drug Delivery of Dexamethasone in Skin Investigated by EPR Spectroscopy, Pharmaceutics, 12 (2020).
[20] S.B. Lohan, S. Saeidpour, A. Solik, S. Schanzer, H. Richter, P. Dong, M.E. Darvin, R. Bodmeier, A. Patzelt, G. Zoubari, M. Unbehauen, R. Haag, J. Lademann, C. Teutloff, R. Bittl, M.C. Meinke, Investigation of the cutaneous penetration behavior of dexamethasone loaded to nano-sized lipid particles by EPR spectroscopy, and confocal Raman and laser scanning microscopy, Eur J Pharm Biopharm, 116 (2017) 102-110.
[21] J. Jager, K. Obst, S.B. Lohan, J. Viktorov, S. Staufenbiel, H. Renz, M. Unbehauen, R. Haag, S. Hedtrich, C. Teutloff, M.C. Meinke, K. Danker, H. Dommisch, Characterization of hyperbranched core-multishell nanocarriers as an innovative drug delivery system for the application at the oral mucosa, J Periodontal Res, 53 (2018) 57-65.
[22] J. Lademann, H. Richter, U.F. Schaefer, U. Blume-Peytavi, A. Teichmann, N. Otberg, W. Sterry, Hair follicles - a long-term reservoir for drug delivery, Skin Pharmacol Physiol, 19 (2006) 232-236.
[23] P. Desai, R.R. Patlolla, M. Singh, Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery, Mol Membr Biol, 27 (2010) 247-259.
[24] J. Lademann, F. Knorr, H. Richter, S. Jung, M.C. Meinke, E. Ruhl, U. Alexiev, M. Calderon, A. Patzelt, Hair follicles as a target structure for nanoparticles, J Innov Opt Heal Sci, 8 (2015).
[25] A. Patzelt, H. Richter, F. Knorr, U. Schafer, C.M. Lehr, L. Dahne, W. Sterry, J. Lademann, Selective follicular targeting by modification of the particle sizes, J Control Release, 150 (2011) 45-48.
[26] A. Teichmann, M. Ossadnik, H. Richter, W. Sterry, J. Lademann, Semiquantitative determination of the penetration of a fluorescent hydrogel formulation into the hair follicle with and without follicular closure by microparticles by means of differential stripping, Skin Pharmacol Phys, 19 (2006) 101-105.
[27] S. Saeidpour, S.B. Lohan, M. Anske, M. Unbehauen, E. Fleige, R. Haag, M.C. Meinke, R. Bittl, C. Teutloff, Localization of dexamethasone within dendritic core-multishell (CMS) nanoparticles and skin penetration properties studied by multi-frequency electron paramagnetic resonance (EPR) spectroscopy, Eur J Pharm Biopharm, 116 (2017) 94-101.
[28] S. Saeidpour, S.B. Lohan, A. Solik, V. Paul, R. Bodmeier, G. Zoubari, M. Unbehauen, R. Haag, R. Bittl, M.C. Meinke, C. Teutloff, Drug distribution in nanostructured lipid particles, Eur J Pharm Biopharm, 110 (2017) 19-23.
[29] S.F. Haag, M. Chen, D. Peters, C.M. Keck, B. Taskoparan, A. Fahr, C. Teutloff, R. Bittl, J. Lademann, M. Schafer-Korting, M.C. Meinke, Nanostructured lipid carriers as nitroxide depot system measured by electron paramagnetic resonance spectroscopy, Int J Pharmaceut, 421 (2011) 364-369.
[30] N. Doge, S. Honzke, F. Schumacher, B. Balzus, M. Colombo, S. Hadam, F. Rancan, U. Blume-Peytavi, M. Schafer-Korting, A. Schindler, E. Ruhl, P.S. Skov, M.K. Church, S. Hedtrich, B. Kleuser, R. Bodmeier, A. Vogt, Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin - assessment by intradermal microdialysis and extraction from the different skin layers, J Control Release, 242 (2016) 25-34.
[31] Y.J. Zhu, C.S. Choe, S. Ahlberg, M.C. Meinke, U. Alexiev, J. Lademann, M.E. Darvin, Penetration of silver nanoparticles into porcine skin ex vivo using fluorescence lifetime imaging microscopy, Raman microscopy, and surface-enhanced Raman scattering microscopy, J Biomed Opt, 20 (2015).
[32] C. Choe, J. Lademann, M.E. Darvin, Confocal Raman microscopy for investigating the penetration of various oils into the human skin in vivo, J Dermatol Sci, 79 (2015) 176-178.
[33] A. Ahluwalia, Topical glucocorticoids and the skin--mechanisms of action: an update, Mediators Inflamm, 7 (1998) 183-193.
[34] A.E. Coutinho, K.E. Chapman, The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights, Mol Cell Endocrinol, 335 (2011) 2-13.
[35] J.U. Junghanns, R.H. Muller, Nanocrystal technology, drug delivery and clinical applications, Int J Nanomedicine, 3 (2008) 295-309.
[36] M. Radtke, A. Patzelt, F. Knorr, J. Lademann, R.R. Netz, Ratchet effect for nanoparticle transport in hair follicles, Eur J Pharm Biopharm, 116 (2017) 125-130.
[37] J. Lademann, H. Richter, A. Teichmann, N. Otberg, U. Blume-Peytavi, J. Luengo, B. Weiss, U.F. Schaefer, C.M. Lehr, R. Wepf, W. Sterry, Nanoparticles - An efficient carrier for drug delivery into the hair follicles, Eur J Pharm Biopharm, 66 (2007) 159-164.
[38] J. Lademann, H. Richter, S. Schanzer, M.C. Meinke, M.E. Darvin, J. Schleusener, V. Carrer, P. Breuckmann, A. Patzelt, Follicular penetration of nanocarriers is an important penetration pathway for topically applied drugs, Hautarzt, 70 (2019) 185-192.
[39] S.H. Kuo, C.J. Shen, C.F. Shen, C.M. Cheng, Role of pH Value in Clinically Relevant Diagnosis, Diagnostics, 10 (2020).
[40] D. Kaden, L. Dähne, F. Knorr, H. Richter, J. Lademann, M.C. Meinke, A. Patzelt, M.E. Darvin, S. Jung, Determination of the pH gradient in hair follicles of human volunteers using pH-sensitive melamine formaldehyde-pyranine Nile blue microparticles Sensors, accepted (2020).

