What is NISSO HPC?
What is NISSO HPC?
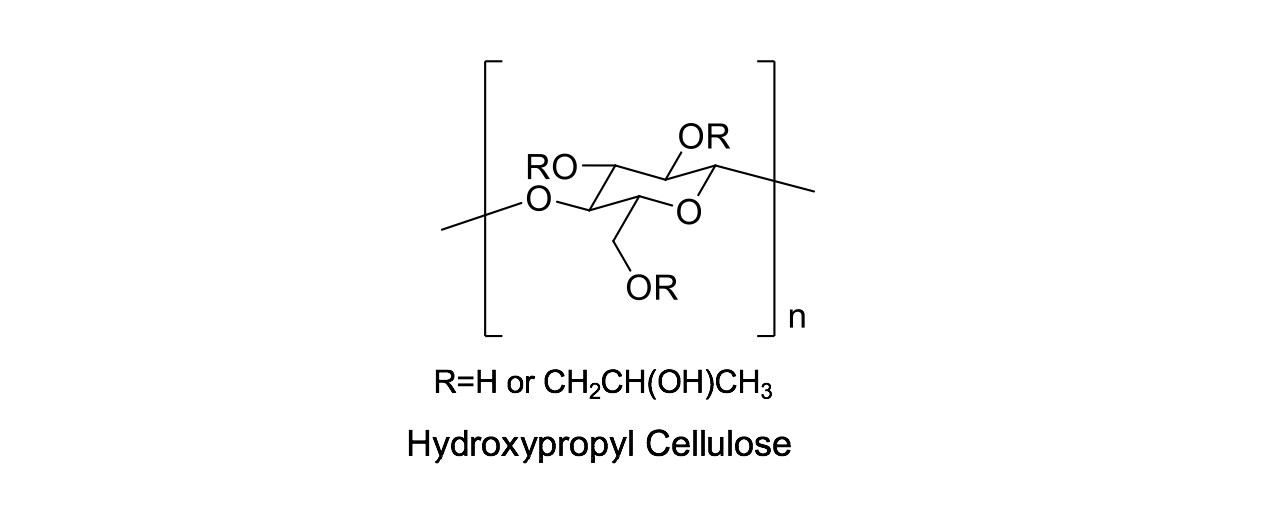
NISSO HPC (Hydroxypropyl Cellulose) is a modified cellulose obtained by reacting propylene oxide with cellulose. The presence of hydroxypropoxy group prevents the hydrogen bonding between the hydroxy groups on the cellulose chain, thereby making HPC soluble. NISSO HPC was first sold in Japan in 1969 and is globally approved for use in food product and as a pharmaceutical excipient. Nippon Soda has multiple manufacturing lines in Japan which have been fully IPEC GMP compliant since 2010.
NISSO HPC grades
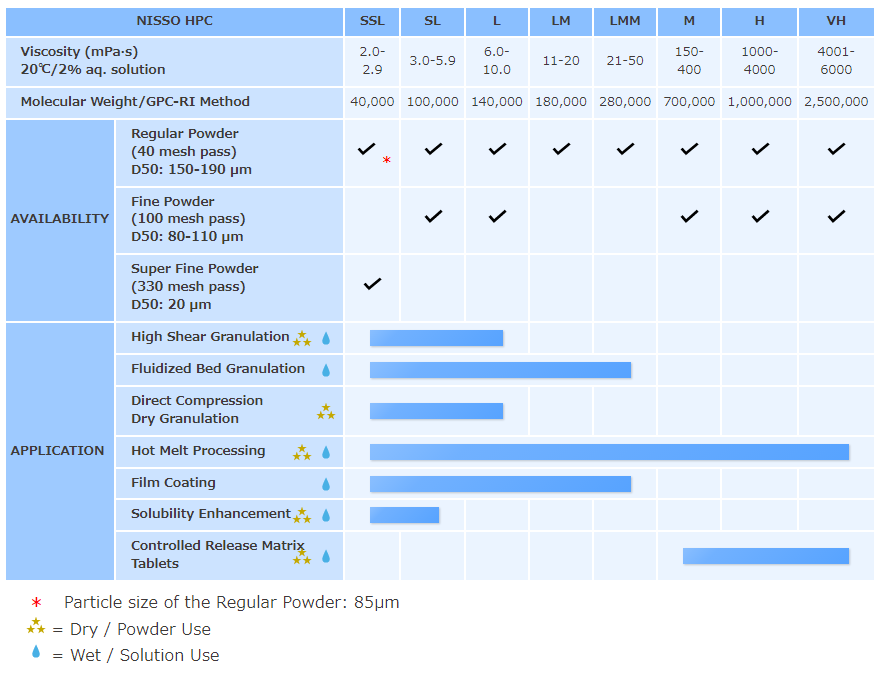
Regular Powder: Regular Powder (RP) type HPC is mainly used in solution form. As a binder for wet granulation applications, low viscosity grades of HPC give an excellent balance of properties to oral solid dosage tablets by imparting superior strength and elegance without compromise of the disintegration profile. As a film coating agent, HPC improves film flexibility, elongation, and adhesion to the tablet.
Fine Powder: Fine Powder (FP) type HPC has better formability than Regular Powder type and is mainly used in dry powder form. Having good wettability, lower viscosity grades are used as a dry-mix binder in high shear mixer formulations. Higher viscosity grades of HPC are applicable to controlled release matrix tablet formulations by direct compression application and give better release profiles compared to other common CR polymers.
Super Fine Powder: Super Fine Powder (SFP) type offers the highest level of formability. Excellent tablet properties can be obtained at low use level in direct compression applications. SFP is applicable to poorly compressible drug and high drug load formulations. Additionally, excellent tablet properties can be achieved in orally disintegration tablet (ODT) formulations by direct compression method when SFP grade is used in combination with a super disintegrator.
How to use NISSO HPC?
Granulation
NISSO HPC can be readily dissolved in both water and most organic solvents to prepare effective granulation solution. Poor flow and compaction actives can be successfully granulated using NISSO HPC to make tableting more feasible through both high shear wet granulation and fluidized bed granulation methods.
Direct compression and Roller Compaction
NISSO HPC is highly plastic and deformable, and exhibits high cohesive and adhesive forces, all important characteristics of a robust direct compression dry binder used for solid oral dosage forms. We often recommend HPC-SSL SFP for some of the most challenging direct compression formulations because of its super fine particle size, high plasticity and enhanced compressibility, allowing formulators to create tablets of outstanding quality.
Controlled release
Higher molecular weight/viscosity grades of NISSO HPC can be used for controlled release hydrophilic matrix tablets. Typical addition rate between 20-30% for effective delayed release of active ingredients are possible. HPC-M,H and VH are recommended.
Case Study
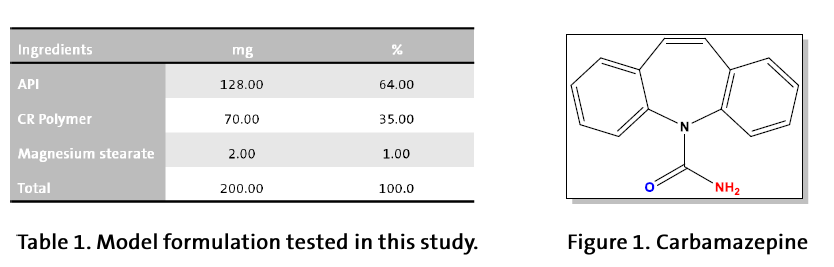
Hydroxypropyl cellulose (HPC) with average aqueous viscosity of 300 mPa.s (NISSO HPC-M FP, d50=110μm) and 3,000 mPa.s (NISSO HPC-H FP, d50=110μm) from Nippon Soda, Japan. Hydroxypropylmethyl cellulose (HPMC) with average aqueous viscosity of 4,000 mPa.s (HPMC K4MCR,d50=100 μm) and 100,000 mPa.s (HPMC K100MCR, d50=100 μm) . A mixture of all ingredients (Table 1) was prepared in a free-fall blender and compressed to 200 mg, 8 mm bi-convex tablets containing 64 % Carbamazepine and 35 % CR polymer. For the dissolution test (USP) we have selected tablets with about 70 N breaking force. The release profile at 100 and 150 rpm was done in purified water as a dissolution medium.
・Powder Compactability
The compaction properties of the HPC-based powder were up to 3 times better compared with the HPMC-based (Figure 2).
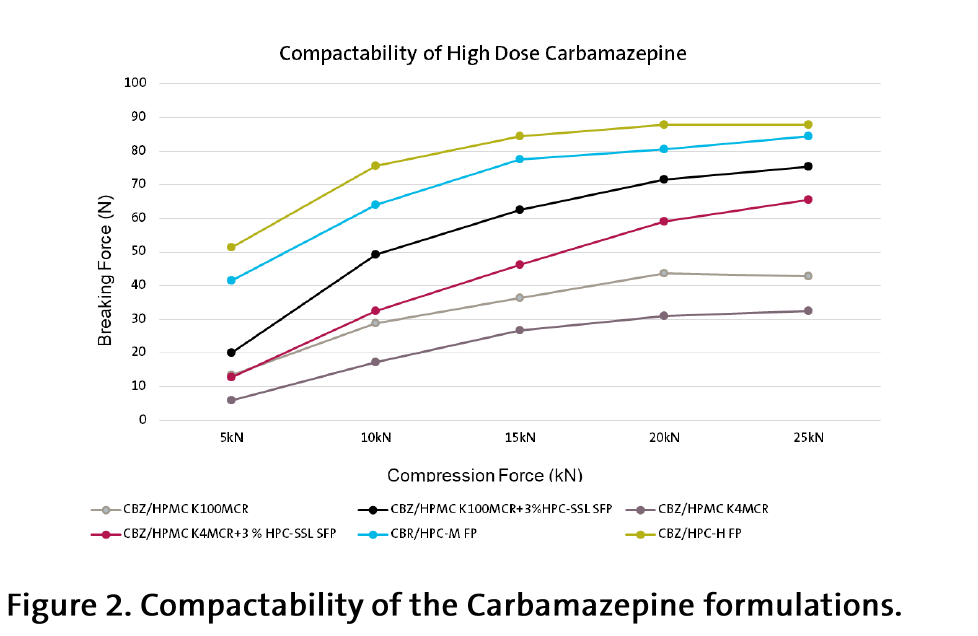
With high drug dose and in absence of binder and filler, the HPC-based tablets demonstrated much better direct compression compactability compared to HPMC. It was necessary to add additionally 3 % of binder (micronized HPC-SSL SFP) in order to get an acceptable breaking forces with HPMC (Figure 2).
・Drug dissolution
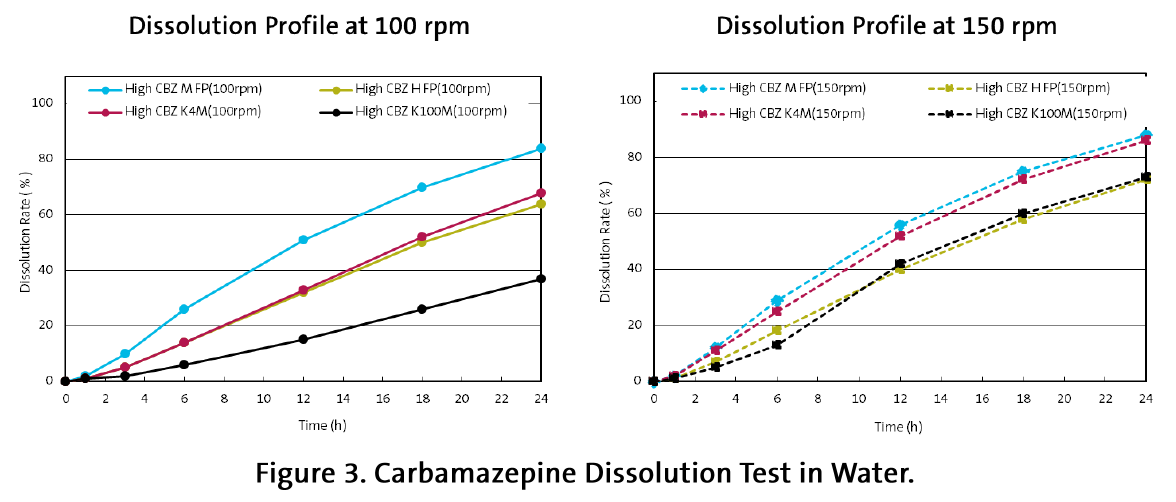
Tablets with breaking force of 70 N (the maximal possible with HPMC K4MCR) were compared in dissolution test in demineralized water (Figure 3).
Being a poorly soluble drug, Carbamazepine (BCS II) release from hydrophilic matrix tablets is based on erosion of the CR polymer. As expected, at 100 rpm, the polymer with the lowest viscosity (HPC-M FP, 300 mPa.s) released the drug fastest that is driven by its quicker erosion/dissolution. The both cellulose ethers with similar viscosity (HPMC K4MCR and HPC-H FP) produced identical drug release and the CR agent with the highest viscosity demonstrated the lowest drug release rate (Figure 3).
With increase of the rotation speed to 150 rpm the picture was changed: whereas the HPC-based formulations showed minor changes in the dissolution profile, the HPMC-based tablets demonstrated a considerable increase of the drug release (Figure 4 and 5).
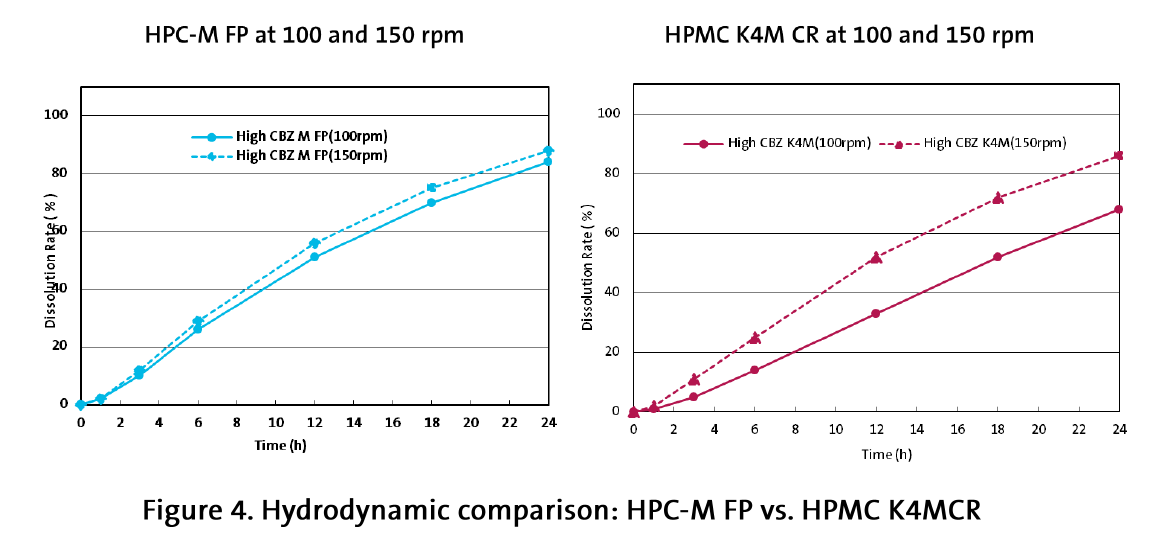
For HPC-M FP, drug release increased only 3-6 % with more hydrodynamic shear (faster ppm), demonstrating consistency and robustness. Comparatively, HPMC K4MCR drug release increased up to 20 % (Figure 4) with more shear.
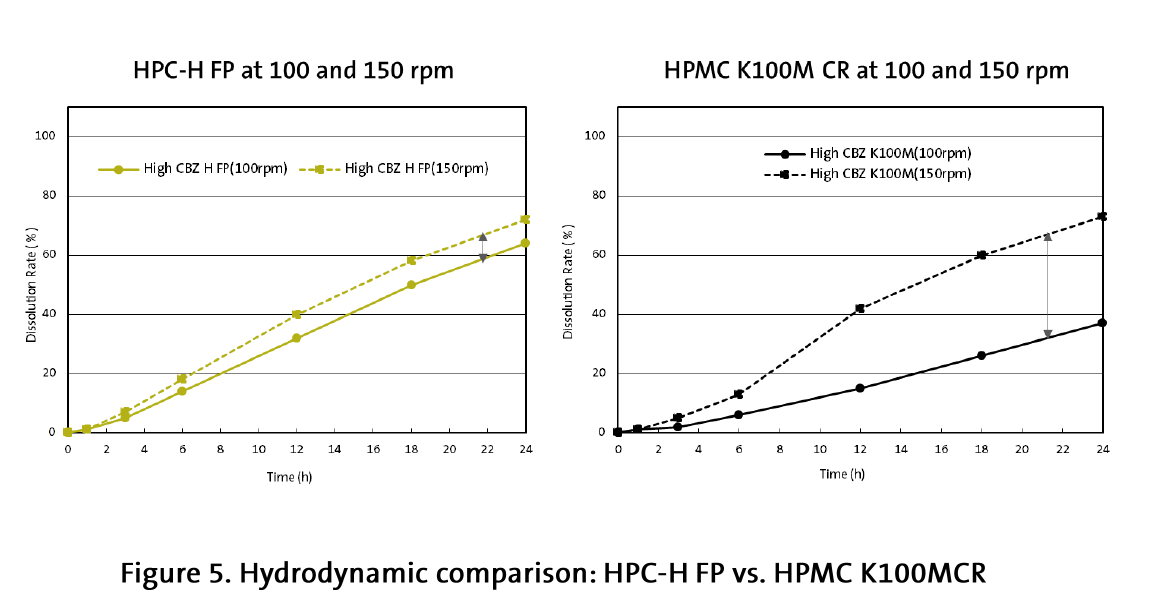
Similar results were obtained when comparing HPC-H FP and HPMC K100MCR. The hydroxypropyl cellulose formulation demonstrated much better dissolution profile consistency and hydrodynamic stability (Figure 5).
・Results and discussion
While the demonstrated better compactability of HPC vs. HPMC can be explained with its superior toughness and plasticity, the better hydrodynamic stability needs further investigations. The numerous hydroxyl groups in the HPC molecule could be responsible for the formation of more new hydrogen bonds after hydration. This doesn’t mean necessarily stronger gel structure but eventually higher resistance to external mechanical stress. Thus, a formation of a more stable gel matrix with HPC provides more robustness under increased hydrodynamic conditions. The presence of methoxyl groups in the HPMC molecule could disturb the formation of multiple inter-molecular hydrogen bonds decreasing in this way the gel network stability compared to HPC.
・Conclusion
High viscosity grades NISSO HPC demonstrated better compactability and hydrodynamic stability compared to HPMC in controlled release hydrophilic matrix tablets with high dose carbamazepine. Additionally, NISSO HPC polymers demonstrated superior dissolution rate consistency and robustness compared to higher viscosity grades HPMC polymers.

