Introduction
Ile-Pro-Pro (IPP) is a bioactive peptide found in bovine milk when casein is hydrolyzed by gastrointestinal enzymes. It has an angiotensin-converting enzyme inhibitory potency of 5µM with the potential to reduce blood pressure, if orally administered.1 Although IPP is stable in intestinal fluids and against Caco-2 cell brush-border peptidases, it has poor intestinal permeability,2 which is the limiting factor in converting it to an oral dosage form. A particular challenge is to find a safe and reversible enhancer to improve epithelial permeability when formulated with peptides and macromolecules. A route of intestinal permeation of IPP may be via the PepT1 carrier, so IPP could be a target for intracellular peptidases. The application of permeation enhancers on the permeability of oral drugs has shown significant results in numerous publications.3 Few publications investigated cellular toxicity of IPP.
The hypothesis was that formulation of IPP in a nanoparticle may localise it near the intestinal wall and increase flux in the context of an incorporated enhancer. The objective of this study was to determine the stability of IPP in rat intestinal fluids and gut homogenates. The permeability of IPP was calculated across rat jejunum and colon mucosae in vitro. Also, the cytotoxicity of IPP in Caco-2 and Hep G2 cells was investigated. These data prepare the way for formulation into nanoparticles for oral delivery
Experimental Methods
Stability of IPP in Homogenates and Gut Washes: A 25 cm section of duodenum/jejunum of male Wistar rats (250–300 g) was isolated. The section was flushed with 10 mL of simulated intestinal fluid (as per USP) to achieve a “gut wash.” The washed section was placed in Hank’s balanced salt solution (HBSS) and homogenized to yield “washed homogenate.” Other sections were placed into HBSS and homogenized to yield “unwashed homogenate.” IPP (4mM) and insulin (200µM) were incubated at 37°C and agitated at 200 rpm. Samples were taken at 0, 30, and 60 min and analyzed by RP-HPLC with the following C18 column gradient mobile phase: A, water 0.05% trifluoroacetic acid; and B, acetonitrile 0.05%. Samples were sourced from three rats and each run in triplicate.
Permeability Across Jejunum and Colon Tissue: Isolated muscle-stripped rat colonic mucosae and unstripped rat jejunal tissue from male Wistar rats were mounted in Ussing chambers. Fluorescein isothiocyanate (FITC)–IPP (500µM) was added to the apical chamber with the addition of 10mM of either sodium caprate (C10) or a novel medium-chain fatty acid derivative (10-undecylenic acid, sodium salt, uC11).4 The apparent permeability (Papp) and transepithelial electrical resistance (TEER) were measured over 120 min. Tissue was sourced from five rats.
Cytotoxicity of IPP Using MTT and MTS Assay: Caco-2 and Hep G2 cells were cultured and seeded on 96-well plates in 200 µL of Dulbecco’s modified Eagle’s medium (DMEM) at 37°C. After 24 h, DMEM was removed and replaced with DMEM with IPP (0.1–10mM) or Triton®-X-100 (0.05%) for 1 or 24 h (Caco-2) or 72 h (Hep G2). Cells were treated with MTT or MTS accordingly. All data were analyzed by one-way ANOVA with Dunnett’s post-test.
Results and Discussion
Incubation of IPP in rat intestinal homogenates and washes showed no evidence of metabolism over 60 min, confirming stability (Figure 1A). Enzymatic capacity of the three systems was confirmed by breakdown of human insulin (Figure 1B). The greatest capacity for metabolism of insulin was the gut wash system, which broke down over 60% in 60 min, compared with 40% with the unwashed homogenate.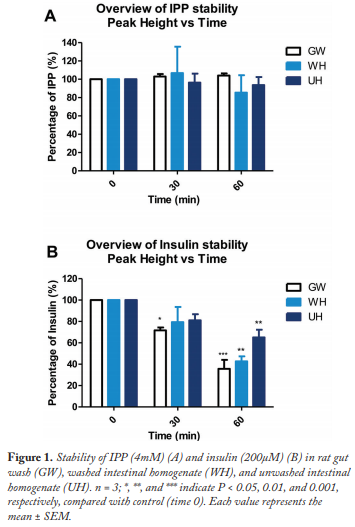
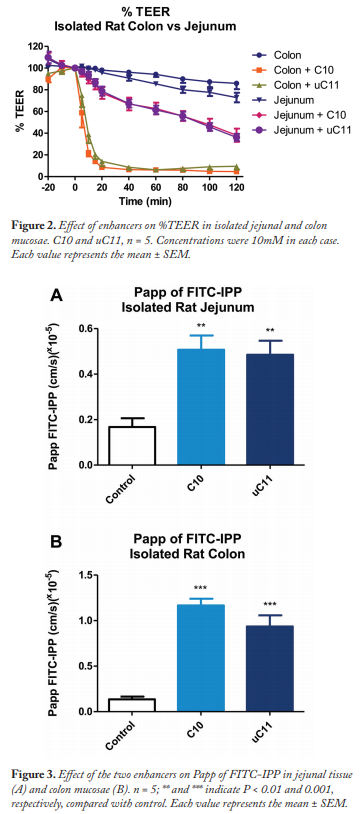
Permeation of FITC-IPP (Figure 2) was slightly higher but not statistically significant in jejunum compared with colon, perhaps because of the higher presence of PepT1 in jejunum. C10 and uC11 increased the Papp of IPP significantly. In parallel, both enhancers significantly (P < 0.001) reduced the TEER from 40 min in jejunum and within 5 min in colon (Figure 2).
Papp of FITC-IPP was significantly increased by medium-chain fatty acid type enhancers in jejunum (Figure 3A) and colon (Figure 3B). These data suggest that the tight junctions are being opened, thereby allowing FITC-IPP to permeate.
Comparison of the MTS and MTT cytotoxicity assays indicated that IPP was not cytotoxic in colon (Caco-2) or liver (Hep G2) cells, even at high concentrations of 10mM for 72 h (Figure 4). This was in agreement with previously published studies performed on CHL cells.
Conclusions
The milk-derived peptide IPP was stable in rat intestinal gut washes and homogenates and was noncytotoxic to human intestinal and liver cells. However, the limiting factor for oral peptide delivery is low intestinal permeability. Permeation enhancers C10 (which is currently in clinical trials) and uC11 (which is used in alternative medicine), were tested successfully with IPP in isolated rat intestinal tissue. This paves the way to investigate the potential of these enhancers in an IPP “nano” construct to potentially reduce blood pressure (Figure 5).
Acknowledgements
This work was supported by Department of Agriculture, Food and Marine under FIRM (Food Institutional Research Measure) Project Ref. 11/F/042.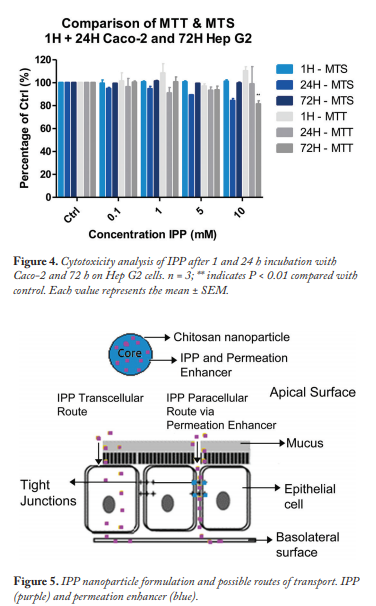
References
1. Nakamura, Y, Yamamoto, N, Sakai, K, Okubo, A, Yamazaki, S, Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk, J. Dairy Sci. 78: 777- 783 (1995). 2. Ohsawa, K, Satsu, H, Ohki, K, Enjoh, M, Takano, T, Shimizu, M. Producibility and digestibility of antihypertensive beta-casein tripeptides, Val-Pro-Pro and Ile-Pro-Pro, in the gastrointestinal tract: Analyses using an in vitro model of mammalian gastrointestinal digestion, J. Agric. Food Chem. 56: 854-858 (2008). 3. Maher, S, Leonard, TW, Jacobsen, J, Brayden, DJ. Safety and efficacy of sodium caprate in promoting oral drug absorption: From in vitro to the clinic, Adv. Drug Deliv. Rev. 61: 1427-1449 (2009). 4. Brayden, DJ, Walsh, E. Efficacious intestinal permeation enhancement induced by the sodium salt of 10-undecylenic acid, a medium chain fatty acid derivative, AAPS J. 16: 1064-1076 (2014).


