CRS members receive a free online subscription
Drug Delivery and Translational Research is a journal published by CRS, providing a unique forum for scientific publication of high-quality research that is exclusively focused on translational aspects of drug delivery. The journal is published 6 times a year and will be available online to CRS members as part of their annual dues.
Access DDTR Online Editor-in-Chief Article Enjoy Articles of DDTR
We welcome research focused on the following areas of translational drug delivery research:
- Designing and developing novel drug delivery systems, with a focus on their application to disease conditions
- Preclinical and clinical data related to drug delivery systems
- Drug distribution, pharmacokinetics, clearance, with drug delivery systems as compared to traditional dosing to demonstrate beneficial outcomes
- Short-term and long-term biocompatibility of drug delivery systems, host response
- Biomaterials with growth factors for stem-cell differentiation in regenerative medicine and tissue engineering
- Image-guided drug therapy
- Nanomedicine
- Devices for drug delivery and drug/device combination products
In addition to original full-length papers, communications, and reviews, the journal will also include editorials, reports of future meetings, research highlights, and announcements pertaining to the activities of CRS.
DDTR Research Editors DDTR Editorial Board
What's New?

Targeted Oral Delivery of Mcroencapsulated TNF-α siRNA in an Experimental Model of Colitis
Summary: This study conducted within UMR 1098 RIGHT and at the University Marie and Louis Pasteur (Besançon, France) demonstrates the therapeutic efficacy of a TNF-α siRNA–based microparticulate technology in an experimental model of colitis. Nucleic acids were first encapsulated in lipid nanoparticles, followed by microencapsulation using a biopolymer. After three consecutive oral administrations, the group treated with the microparticles showed a highly significant improvement in clinical symptoms, a reduction in macroscopic intestinal lesions, and a decrease in the overall inflammatory response. Biodistribution studies demonstrated specific targeting of inflamed intestinal lesions. This work paves the way for a new oral RNA-based therapeutic strategy dedicated to the treatment of chronic inflammatory bowel diseases.

Title: Mitigating radioresistance mechanisms by polymer-lipid manganese dioxide nanoparticles enhances immunogenic cell death and antitumor immune response to facilitate abscopal effect in breast tumor models
Link: https://link.springer.com/article/10.1007/s13346-025-01873-1

Preparation, characterisation, and testing of reservoir-based implantable devices loaded with tizanidine and lidocaine
Summary: This study describes the design and formulation optimisation of core-shell implantable devices for the delivery of two drugs: tizanidine and lidocaine. The implant comprises a core loaded with tizanidine and a porous membrane to control its release. Both drugs were incorporated separately into distinct regions of the device. All materials used in the fabrication of the implant were biodegradable. Various formulations were evaluated to sustain the delivery of tizanidine over prolonged periods, aiming to manage spasticity in conditions such as multiple sclerosis. Lidocaine, on the other hand, was incorporated into the porous membrane to enable short-term release, intended to alleviate the pain associated with implant administration. This pain is one of the limitations commonly encountered with subcutaneous implant delivery. The inclusion of lidocaine in the membrane could potentially be applied to other formulations, enabling the development of alternative core-shell implants for the long-term delivery of different drugs loaded in the core.

Intranasal delivery of engineered anti-SARS-CoV-2 extracellular vesicles therapeutically represses lung infection and inflammation
Summary: We report a first-in-vivo demonstration that intranasally delivered, engineered extracellular vesicles (EVs) carrying a conserved anti-SARS-CoV-2 nanobody (VHH72) can blunt lung infection and dampen inflammation in a mouse model of COVID-19. These neural-stem-cell–derived EVs are “armored” by displaying the nanobody on their surface, enabling direct binding to the viral spike protein and neutralization of infectious particles. Infected mice treated intranasally showed ~2-log reductions in lung viral load, alongside transcriptomic signatures consistent with reduced cytokine signalling. The work underscores two ideas with broad relevance: (1) the respiratory tract can be targeted non-invasively via the nose, and (2) modular EVs can be built as dual-action antivirals that combine virus neutralization with immune calming. We see this platform as a stepping stone toward rapid, variant-agnostic antivirals for future outbreaks.

Summary: Despite progress in the development of nanomedicines delivering nucleic acid therapeutics, their clinical translation is still hampered by multiple obstacles. Herein, a novel targeting strategy was adopted via tweaking the composition of the nanocarrier to manipulate the endogenous protein corona adsorbed to it post administration so that endogenous pathways are recruited for specific delivery of the cargo to the target cells without the need for external targeting ligands. Lipid nanoparticles (LNPs) with simple composition were designed based on a library of ionizable lipids. Optimizing the composition of the LNPs maximized their tropism to the spleen by 20-fold compared to the liver and successfully transfected the antigen-presenting cells (APCs) after intravenous administration. Loading the LNPs with a low dose of antigen-encoding mRNA protected mice from tumor challenges and proved to be safe upon either acute dose escalation or repeated administrations. This scalable platform is promising for translation as a neoantigen vaccine.



Summary:
- Summary: Clearance by the immune system and inefficient tumor targeting are outstanding challenges associated with nanomedicine, such as liposomes. In this paper, the authors propose a novel strategy to achieve precise targeted treatment of malignant tumors. Liposomes are fused with extracted natural cell membranes and artificial phospholipids to reassemble a composite nanolipid carrier (recombined lipid nanocarriers (RLNs)). This work characterizes different types of cell membrane composite lipid nanocarriers and demonstrates enhanced tumor targeting, promising antitumor effects and safety profiles.

Summary: This collaborative work between UMC Utrecht with Radboud UMC and Maastricht University explores a novel approach to safely deliver RNA-based therapeutics during pregnancy. We demonstrated that targeted lipi nanoparticles (LNPs), functionalized with VHH nanobodies, remained within the maternal circulation without crossing into the fetal bloodstream. The findings were confirmed using an ex vivo human placenta perfusion model and multiphoton microscopy. This result forms a significant step toward developing safe, site-specific drug delivery systems for pregnant patients.

Pneumolysin-responsive liposomal platform for selective treatment of Streptococcus pneumoniae
Summary:
- Summary: Streptococcus pneumoniae has become a leading cause of meningitis, sepsis, and bacterial pneumonia worldwide, with increased prevalence of antibiotic-resistant serotypes serving to exacerbate the issue. The main factor responsible for colonization and immune response escape in pneumococcal infections is the secreted molecule pneumolysin, which is a subset within a family of related toxins that form transmembrane pores in biological membranes. The present work aimed to examine the impact of liposomal cholesterol content for pneumolysin-induced release of the encapsulated antimicrobial peptide nisin. It was determined that a cholesterol content above 45 mol% was necessary to facilitate interactions with both purified pneumolysin toxin and S. pneumoniae culture. Antibacterial testing highlighted the ability of the developed platform to elicit a potent and specific bactericidal response in vitro against cultured S. pneumoniae when compared to a control strain, Staphylococcus epidermidis. It further improved viability of a fibroblast cell line upon S. pneumoniae challenge, outperforming free nisin.

Flexible surface acoustic wave technology for enhancing transdermal drug delivery
Summary: This study develops a flexible acoustic wave patch for transdermal delivery of dextran macromolecules with various molecular sizes into agarose gels and porcine skin. We have achieved the maximum delivery depths of 1.1 mm (4 kDa) in agarose gel, and ~100 kµm (4 kDa) and ~25 µm (2000 kDa) in porcine skin. The key mechanisms were identified as mechanical agitation, localized streaming, and acousto-thermal effects, with also contribution from micro/nanoscale acoustic cavitation. Such the acoustic wave enhanced transdermal drug delivery depends on wave frequency and intensity, duration of applied acoustic waves, temperature, and drug molecules' molecular weight. It offers a promising solution for active, effective, and precise non-invasive drug administration.
March 2025 Best Paper in Drug Delivery and Translational Research

Delivery of exogenous miR-19b by Wharton’s Jelly Mesenchymal Stem Cells attenuates transplanted kidney
: Renal ischemia-reperfusion injury (IRI) frequently occurs following kidney transplantation, and exosomes derived from umbilical cord mesenchymal stem cells (WJ-MSC-Exos) have shown promise in treating IRI in transplanted kidneys. The study demonstrates that abundantly present miR-19b in WJ-MSC-Exos prevents IRI in transplanted kidneys, while its absence abolishes the protective effects of WJ-MSC-Exos against renal IRI. Mechanistically, miR-19b suppresses glycogen synthase kinase-3β (GSK3β) expression, thereby stabilizing PDXK protein through direct binding. Treatment with WJ-MSC-Exos led to reduced PDXK levels and enhanced pyridoxine accumulation, ultimately mitigating IRI in transplanted kidneys and I/R-induced HK2 cell apoptosis. By elucidating the underlying mechanism of WJ-MSC-Exos, the study unveils novel therapeutic targets for post-kidney transplantation IRI and also demonstrates the potential for the clinical application of WJ-MSC-Exos in IRI treatment post-transplantation.
Locally administered nanosuspension increases the delivery of estradiol for the treatment of vaginal atrophy in mice
Vaginal atrophy, affecting up to 57% of post-menopausal women, causes symptoms like vaginal burning and dysuria. Estradiol hormone replacement therapy is often prescribed, but traditional vaginal products may lead to side effects such as increased discharge and local tissue toxicity due to hypertonic formulations. We instead developed a novel estradiol nanosuspension (NS) formulation coated with Pluronic F127 for enhanced vaginal delivery. Compared to the standard Estrace cream, this formulation demonstrated improved estradiol delivery to vaginal tissues in pharmacokinetic studies. Using a murine model of post-menopausal vaginal atrophy (ovariectomized mice), the study showed equivalent efficacy in promoting vaginal re-epithelialization with the estradiol NS and Estrace cream. Additionally, the estradiol NS was compatible with vaginal bacteria in vitro. These findings suggest that the Pluronic F127-coated estradiol NS is a promising alternative treatment for post-menopausal vaginal atrophy, potentially offering improved safety and effectiveness over existing therapies.


January Best Paper

Local Delivery of Doxorubicin Prodrug via Lipid Nanocapsule-based Hydrogel for the Treatment of Glioblastoma
Summary: Our study introduces a novel combinatory treatment for glioblastoma, aimed at targeting residual tumor cells and reducing post-surgical inflammation to delay recurrence between surgery and the start of standard care chemoradiation. The lauroyl-doxorubicin lipid nanocapsule hydrogel (DoxC12-LNC) features spontaneous gelation, injectability, sustained drug release and mechanical properties optimized for intracerebral delivery. In an orthotopic GL261 glioblastoma resected mouse model, this local treatment shows therapeutic efficacy, which is further enhanced by post-surgical systemic administration of ibuprofen.
Realizing time-staggered expression of nucleic acid-encoded proteins by co-delivery of messenger RNA and plasmid DNA on a single nanocarrier
Unlocking the Potential of Nanomedicine: Advances in Precision Targeting Strategies
RGD-coated polymeric microbubbles promote ultrasound-mediated drug delivery in an
inflamed endothelium-pericyte co-culture model of the blood-brain barrier
https://link.springer.com/article/10.1007/s13346-024-01561-6
Drug delivery to the brain is limited by the blood-brain barrier (BBB). Sonopermeation, which
combines ultrasound (US) and microbubbles (MBs), is a promising strategy to temporarily
open the BBB and enhance drug delivery. We developed an advanced in vitro BBB model
using human cerebral endothelial cells and pericytes to study the effects of sonopermeation
on the delivery of a large polymeric drug carrier (pHPMA) and a small molecule drug
(ribavirin) under normal and inflamed conditions. By measuring trans-endothelial electrical
resistance (TEER), we found that sonopermeation significantly reduced the TEER values
and increased the translocation of pHPMA. Inflammation was induced with tumor necrosis
factor, mimicking the conditions in tumors and CNS infections. RGD-coated MBs specifically
targeted inflamed endothelium, enhancing the delivery of both pHPMA and ribavirin across
the BBB. Our study demonstrates how combining in vitro BBB models with targeted
sonopermeation can optimize drug delivery to the (inflamed) brain, laying the foundation for
future research.
September 2024

Nose to brain delivery of mirtazapine via lipid nanocapsules: Preparation, statistical optimization, radiolabeling, in vivo biodistribution and pharmacokinetic study
https://link.springer.com/article/10.1007/s13346-024-01528-7
Mirtazapine (MZP) is an FDA-approved antidepressant drug but suffer from low bioavailability, which is only 50% despite its rapid absorption after oral administration. This study seeks to deliver MZP intranasally to the brain via lipid nanocapsules (LNCs). MZP-LNCs were constructed by solvent-free phase inversion temperature technique applying D-Optimal mixture design to study the impact of formulation variables, including percentage of Labrafac oil, Solutol, and water, on the characterization of the formulated nanocapsules. The optimized MZP-LNCs exhibited spherical morphology, particle diameter of 20.59 nm, z-potential of -5.71 mV, PDI of 0.223, and solubilization capacity of 7.21 mg/g. The in vivo pharmacokinetics of the intranasally administered MZP-LNCs in brain and blood were correlated with intravenously and intranasally administered MZP solution in mice. In vivo biodistribution analysis revealed that MZP-LNCs were able to deliver greater MZP amount to the brain, with lesser blood MZP levels, compared to MZP solution. Overall, MZP-LNCs showed greater nose-to-brain delivery compared to MZP solution.
The selection of animal models influences the assessment of anti-tumor efficacy:
promising sialic acid-conjugate modified liposomes demonstrate remarkable therapeutic
effects in diverse mouse strains
https://link.springer.com/article/10.1007/s13346-023-01502-9
Mouse models serves as a crucial tool for preclinical assessment of antineoplastic agents, but
the impact of physiological differences among mouse strains on in vivo antitumor efficacy has
been overlooked. Mononuclear phagocyte system (MPS) is the major player in clearance in
vivo, and differences in MPS among different strains may potentially impact the effectiveness of
antitumor preparations. We thus employed conventional liposomes (CL-EPI) and sialic acid-
octadecamine-modified liposomes (SAL-EPI) as model preparations to compare the therapeutic
effects in tumor-bearing KM, BALB/c, and C57BL/6 mice. The results demonstrated significant
variability in the efficacy of CL-EPI for tumor treatment across different mouse strains,
necessitating careful selection of animal models. SAL-EPI effectively targeted tumor sites by
binding to Siglec-1 on the surface of peripheral blood monocytes (PBMs) and achieved
uniformly improved therapeutic effect in different mouse strains. The sialic acid-modified
preparation is therefore expected to achieve a favorable therapeutic effect in tumor patients with
different immune states through PBMs delivery (Siglec-1 was expressed in both mice and
humans), thereby possessing clinical translational value and promising development prospects.
The Innovation of Microneedle Technologies for Drug Delivery Applications:
November 2023 Issue
Intravenous injection of cyclosporin A loaded lipid nanocapsules fights inflammation and immune system activation in a mouse model of diabetic retinopathy).
https://link.springer.com/article/10.1007/s13346-023-01350-7
Summary:
Inflammation and immune system activation are key pathologic events in the onset and
escalation of diabetic retinopathy (DR). Both are driven by cytokines and complement
originating from the retinal pigment epithelium (RPE). Despite the RPE’s pivotal role, there is no
therapeutic tool to specifically interfere with the RPE-mediated pathology of DR. Here, we
utilized lipoprotein-mimetic lipid nanocapsules to deliver the anti-inflammatory and
immunosuppressive drug cyclosporin A (CsA) to RPE cells. Using a mouse model of DR that
mirrors all pathologic aspects of human DR, we demonstrate that intravenously applied CsA-
loaded lipid nanocapsules comprehensively counteract inflammation and immune system
activation. One single injection suppressed the expression of pro-inflammatory cytokines,
dampened macrophage infiltration, and prevented macrophage and microglia activation in eyes
with DR. This work shows that CsA-loaded lipid nanocapsules can offer new avenues for the
treatment of DR.
Nebulizers effectiveness on pulmonary delivery of alpha1 antitrypsin
https://link.springer.com/article/10.1007/s13346-023-01346-3
Summary:
The administration of nebulized alpha-1 antitrypsin (AAT) to the lung represents an interesting
alternative to parenteral infusion for patients suffering from AAT genetic deficiency (AATD).
However, nebulization of proteins can have a dramatic effect on their activity. In this paper, a
formulation of AAT for infusion was nebulized with a jet or a mesh vibrating system, whose
performance was compared in terms of aerosolization efficiency and preservation of AAT function.
The two nebulizers allowed a satisfactory preservation of protein activity and provided equivalent
aerosolization performances, while the delivered dose was higher with the mesh vibrating system.
Nebulization of AAT proved to be a suitable administration strategy ready to be translated to the
clinical practice for direct lung delivery of the protein in AATD patients, either as a support therapy
to parenteral administration or, for subjects with a precocious diagnosis, as a means to prevent the
onset of pulmonary symptoms.
Neuronal differentiation and functional maturation of neurons from neural stem cells
induced by bFGF-chitosan controlled release system
https://link.springer.com/article/10.1007/s13346-023-01322-x
Summary:
Available methods for differentiating stem cells into functional neurons require numerous
cytokines and neurotrophic factors, and the process is highly complex, slow, inefficient, and
costly for clinical implementation. Here, we demonstrate a bioactive material, basic fibroblast
growth factor (bFGF)-chitosan controlled release system, facilitates neuronal differentiation from
neural stem cells (NSCs) and the functional maturation of the induced neurons with high
efficiency. Immunostaining revealed that the neurons derived from NSCs expressed mature
immunomarkers of interneurons and excitatory neurons. We also found by patch-clamp that the
induced neurons exhibited diverse electrophysiological properties as well as functional
synapses. In vivo, we implanted bFGF-chitosan into lesion area in traumatic brain injury mice
and observed abundance of neuroblasts in subventricular zone and the presence of newborn
functional neurons in injury area, which integrated into synaptic networks. Taken together, our
efficient and rapid tissue engineering approach provides a means to generate functional
neuronal lineage cells from stem cells and potentially to treat brain injury and diseases.
Drug Delivery and Translational Research – Best Paper August 2023 Issue
Summary:
https://link.springer.com/article/10.1007/s13346-022-01190-x
The discovery of proteins that neutralize vascular endothelial growth factors can inhibit the
process of angiogenesis to restore eyesight in individuals with retinal vascular disorders.
However, a safe and effective means to deliver these protein drugs to the target posterior
segment is currently lacking. To this end, we developed dissolving bilayer microneedles (MNs)
possessing the potential to deliver proteins to the back of the eye in an efficient and minimally
invasive manner. A model protein, ovalbumin (OVA), was incorporated into MNs fabricated from
different polymers, including hyaluronic acid, polyvinyl alcohol (PVA) and polyvinylpyrrolidone
(PVP). Optimized PVA/PVP MNs demonstrated robust permeation of porcine sclera with > 75%
of the needle length penetrating the sclera while dissolving within 150 s. SDS-PAGE and OVA-
specific ELISA revealed that the bioactivity of OVA was retained during the manufacture of MNs.
In hen’s egg-chorioallantoic membrane test, MNs fabricated from all chosen polymers were
classified as non-irritants. Furthermore, ex vivo permeation studies showed that optimized MNs
mediated the penetration of 86.99 ± 7.37% of OVA through the sclera, twice that of the needle-
free patch (42.16 ± 3.95%), highlighting the capability of MNs to circumvent physical barriers
and promote protein delivery to the posterior segment of the eye. Overall, a novel, efficient, and
safe intraocular protein delivery system was successfully established.
Drug Delivery and Translational Research – Best Paper July 2023 Issue
Summary:
https://link.springer.com/article/10.1007/s13346-023-01319-6
Despite recent clinical successes of the chimeric antigen receptor T cell therapy in treating liquid
cancers, numerous challenges hamper its broader translation. Macrophage has been proposed
as an alternative given its abilities in promoting tumor infiltration, acquiring diverse antigens, and
continuously stimulating adaptive responses. However, poor survival of transplanted
macrophages and transient retention of anti-tumor phenotype have been major obstacles.
Leveraging on recent discoveries of nanoparticle strategies addressing these limitations, we
herein report enhanced survival and phenotypic retention of macrophage transplants in murine
lungs by pre-treatment with nanoparticles of varying degradation rates. Poly(ethylene glycol)
diacrylate nanoparticles prolonged the survival of transplanted macrophages over untreated cells,
where nanoparticles increased the retention of transplanted cell counts by over 50%.
Furthermore, pre-treated macrophages more efficiently retained the pro-inflammatory-like
polarization state compared to macrophages pre-treated with a classical pro-inflammatory
stimulus, interferon-gamma, where CD86 costimulatory molecule expression was over 150%
greater in pre-treated macrophage transplants compared to untreated counterparts. These
findings provide an avenue for a major improvement in the lifespan and efficacy of macrophage-
based therapies and thus their broader therapeutic implementation.
Drug Delivery and Translational Research – Best Paper June 2023 Issue
Summary:
Sustained antigens delivery using composite microneedles for effective
epicutaneous immunotherapy Allergen-specific immunotherapy is an efficacious therapy for various allergic diseases such as
food allergy (FA). However, frequent clinical visits and potential adverse effects often hinder
patient compliance. Here, we proposed an implantable microneedle (MN) system composed of
OVA (antigen)-loaded silk MNs and a dissolvable, flexible polyvinyl alcohol (PVA) pedestal.
Once MNs are inserted into the skin, the PVA pedestal can quickly dissolve in the interstitial
fluid of the excised skin and implant the OVA-loaded silk MN tips in dermal layer as a sustained
antigen depot, thus inducing a long-lasting immune response. After receiving 3 doses of MN-
based immunotherapy, the immune response in OVA-sensitized mice was successfully
suppressed, with no apparent side effects. Compared to conventional subcutaneous
immunotherapy, MN immunotherapy ameliorated systemic anaphylaxis more effectively even at
a lower dose, demonstrating the antigen dose-sparing potential of the proposed MNs. Moreover,
due to the prolonged release effect of silk-PVA composite MNs, the frequency of
immunotherapy can be significantly reduced. Overall, through prolonged skin exposure to
antigen, this implantable designed MN may offer a new therapeutic strategy for FA treatment
with significant improvements in efficacy and convenience.
Drug Delivery and Translational Research – Best Paper May 2023 Issue
Summary:
Preterm-born infants are often susceptible to necrotizing enterocolitis (NEC), a terrible disease associated with impaired intestinal barrier properties, and are often empirically treated with intravenous broad-spectrum antibiotics. Yet, it is unclear how this antibiotic exposure, as well as the way they are dosed, affects the intestinal barrier and minimizes the risk of developing NEC. We show that permeability rates through intestinal mucosa from piglets dosed with the combination of enteral and parenteral antibiotics were comparable to the rates in untreated piglets, whereas the piglets dosed with parenteral antibiotics had lower permeability rates. Interestingly, decreased permeability rates through the mucus alone were evident, suggesting that the change in the mucosa permeability to a large extent was caused by altered mucus permeability. Mixing the antibiotics with untreated mucus had no effect, suggesting that the altered properties of mucus were caused by other associated events induced by the antibiotics.
Drug Delivery and Translational Research – Best Paper April 2023 Issue
Summary:
The analysis of gastrointestinal cellular permeation in pre-clinical drug development is widely
conducted, however the permeation of the overlying mucus layer is often overlooked. This is
attributed to a lack of understanding of the complexity of this layer, coupled with minimal
availability of experimental tools to quantify permeation through the turbid, viscous solution. Here,
a novel microfluidic device facilitates the observation of interactions between physicochemically
diverse nanocarriers and the mucin protein on a microscopic scale. This approach provides key
information on mucin-binding which may be lost with traditional methods and allows the
quantification of mucoadhesive as well as mucopermeating formulations. In addition, utilising a
complex biomimetic solution of proteins, lipids and salts which accurately mimics the chemistry and
rheology of native mucus within the device resulted in a 5.5-fold reduced permeation and 1.4-fold
reduced diffusivity of PLGA nanoparticles, compared to a more prevalent, simple mucus mimic.
Summary: Circular RNAs (circRNAs) are a class of highly stable and closed-loop noncoding RNA that are
involved in the occurrence and development of hepatocellular carcinoma (HCC). We found that
high circ_0058051 expression was negatively correlated with the prognosis of HCC patients.
We also showed here that circ_0058051 knockdown attenuated the proliferation and colony
formation, while inhibited migration of HCC cells. The findings underscore the potential of
circ_0058051 as a therapeutic target for HCC. We synthesized a novel small interfering RNA
(siRNA) delivery system, PEG-PCL-PEI-C14-SPIONs (PPPCSs), based on superparamagnetic
iron oxide nanoparticles (SPIONs). PPPCSs protected the siRNA of circ_0058051 from
degradation in serum and effectively delivered siRNA into SMMC-7721 cells. Meanwhile,
intravenous injection of the PPPCSs/siRNA complex could inhibit tumor growth in the
subcutaneous tumor model. In addition, the nanocomposite was not toxic to the organs of nude
mice. Collectively, PPPCSs/si-circ_0058051 complex may provide a novel and promising
method of HCC treatment.
Best Papers of the February 2023 Issue of DDTR (listed by corresponding authors):
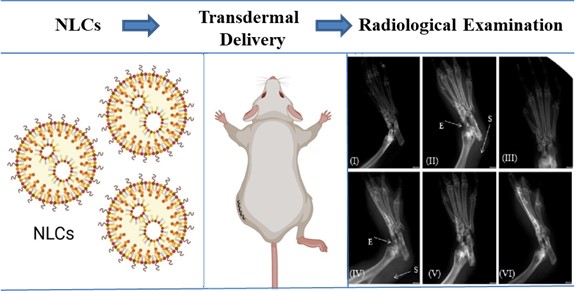
Summary: Thymoquinone (TQ) is a quinone-based phenolic compound with antioxidant and anti-
inflammatory activities, but its therapeutic utility has been underexplored due to inadequate
biological stability, short half-life, low hydrophilicity, and poor systemic bioavailability.
Tamanu oil-stabilized nanostructured lipid carriers (TQ-NLCs) enriched with TQ were
prepared and optimized using Box-Behnken design with the size of 153.9 ± 0.5 nm and
surface charge of -30.7 mV. The encapsulation efficiency and drug loading density were
found to be 84.6 ± 0.5 % and 14.8 ± 0.5 %, respectively. The TQ-NLCs assayed for skin
permeation for transdermal delivery where TQ-NLC provided roughly 15 times greater
permeation compared to aqueous solution of TQ. Tamanu oil displayed a synergistic anti-
inflammatory potential with TQ in comparison to TQ alone in carrageenan-induced paw
oedema model and Freund's adjuvant-induced arthritic model. The arthritic and X-ray scores
significantly reduced in TQ-NLC-treated and standard formulation-treated groups. Moreover,
serum pro-inflammatory TNF-α and IL-6 levels were significantly reduced in TQ-NLC-treated
group compared to the arthritic control group.
Best Papers of the December 2022 Issue of DDTR (listed by corresponding authors):
Summary: We introduce a novel antiviral nano-drug called SNAT (Smart Nano-Enabled
Antiviral Therapeutic) composed of taxoid (Tx)-decorated amino (NH 2 )-functionalized silver
nanoparticles (Tx–[NH 2 -AgNPs]). The particles are around 5 nm in diameter, positively charged
and stable for over three years at room temperature. We assessed the preclinical efficacy of
inhaled SNAT where we found that SNAP significantly reversed the body weight loss, reduced
the virus load in oral swabs, and improved lung health of hamsters infected with SARS-CoV-2.
Further, SNAT was found to be noncytotoxic and antioxidant, potentially quenching lipid
peroxidation, in human lung epithelial cells and dermal fibroblasts. Overall, our study
collectively highlights SNAT as a safe and potent antiviral platform against SARS-CoV-2
infection and potentially other respiratory viruses of epidemic and pandemic potential.
Summary: A potent therapeutic option is sorely needed for triple negative breast cancer (TNBC) due to its clinical concerns, such as poor prognosis, drug resistance, and tumor recurrence. We here explored anti-cancer potential of synthetic cannabidiol (CBD; GLP grade) as a monotherapy or a chemosensitizer for doxorubicin (DOX) in TNBC (i.e., MDA-MB-231 and MDA-MB-468) cells. In comparison to 2D cultures, CBD showed greater IC 50 values in hydrogel-based 3D cultures of MDA-MB-231 and MDA-MB-468 cells. Next-generation RNA sequencing revealed GADD45A, GADD45G, FASN, LOX, and integrin (i.e., -α5, -β5) genes to be novelly altered by CBD in MDA-MB-231 cells. CIM-16 plate-based migration assay and western blotting analysis disclosed that CBD induced anti-migratory effects in TNBC cells. Western blotting, RT-qPCR, and immunocytochemistry revealed that CBD inhibited autophagy of TNBC cells. CBD pre-treatment increased DOX sensitivity in TNBC cells. CBD pre-treatment accompanied by DOX treatment decreased LOX, integrin-α5, and increased caspase 9 respectively in MDA-MB-468 cells.
Maria Jose Alonso: Quantification of the actual composition of polymeric nanocapsules: a quality control analysis
Summary: The exact compositions of nanocarriers are rarely described in the literature. However, this information is critical for understanding and/or predicting the biological behaviors of the nanocarriers. This paper describes for the first time the exact composition of polymeric nanocapsules using liquid chromatography-mass spectrometry methodology and the results shows that actual composition is significantly different from the theoretical one. While the work focuses on polymeric nanocapsules, this methodology can be broadly applicable to other nanosystems and would be crucial for understating protein corona formation and targeting capability of functionalized nanocarriers.
Perspective

Role of drug delivery technologies in the success of COVID-19 vaccines: a perspective
This newly published DDTR “Perspective” article discusses the roles of drug delivery technologies in developing safe and efficacious vaccines. We thank Drs. Robert Langer, Pieter Cullis, Olivia Merkel and Mark Prausnitz for sharing their perspectives and insights on this important topic.
DDTR Videos
Editor-in-Chief María José Alonso, PhD talks about DDTR, an official journal of the CRS.
Prof. Ben Boyd, President of the CRS and Editor of DDTR, talks about his experience with the DDTR, an official journal of the CRS.
Inspirational Note

This newly released Inspirational Note discusses critical considerations for the clinical implementation of nanomedicine.

Inspirational note by Martin J. Whitaker, Hiep Huatan and Richard J. Ross
The newly published Inspirational Note features innovative drug delivery technology that replicates the physiological cortisol diurnal rhythm for chronotherapy.

Inspirational note by James Goodson and Dr. Paul Rota
In this inspirational note, James Goodson and Dr. Paul Rota provide insightful and timely perspective and discussion regarding the importance of vaccine delivery technology and multidisciplinary efforts to achieve global vaccination and ultimately well-being.

Inspirational note by Dr. Andrew L. Lewis
Development and approval of rybelsus (oral semaglutide): ushering in a new era in peptide delivery
In this Inspirational Note, Dr. Andrew Lewis and coauthors feature an amazing and innovative discovery that is changing the paradigm of oral peptide delivery.

2020 DDTR Best Paper of the Year: Translational studies of intravenous and intracerebroventricular routes of administration for CNS cellular biodistribution for BMN 250, an enzyme replacement therapy for the treatment of Sanfilippo type B.
2019 DDTR Best Paper of the Year: Depletion of collagen by losartan to improve tumor accumulation and therapeutic efficacy of photodynamic nanoplatforms

2019 CRS Nanomedicine and Nanoscale Delivery (NND) Best Paper of the Year: Tumor growth inhibition by mSTEAP peptide nanovaccine inducing augmented CD8+ T cell immune responses

2019 DDTR CRS Oral Drug Delivery Focus Group Paper of the Year Award: Loratadine self-microemulsifying drug delivery systems (SMEDDS) in combination with sulforaphane for the synergistic chemoprevention of pancreatic cancer
Read DDTR's First Inspirational Note: Contraceptive technologies for global health: ethically getting to safe, effective and acceptable options for women and men
ISSN: 2190-3948
Editor-in-Chief:
Maria José Alonso, PhD - University of Santiago de Complotela, Spain


