Locteron® is an injectable sustained release formulation of interferon-a2b that is one of very few sustained release protein formulations to reach late-stage clinical trials. It is currently looking to be an excellent example of the advantages of controlled release technologies and the positive impact they can potentially have on patients’ lives. Andy Lewis (Editor-in-Chief of the CRS website) interviewed Ruud Verrijk (Chief Scientific Officer, OctoPlus) on its development from concept to the clinic.
Q What makes interferon-a an attractive candidate for an injectable sustained release formulation?
A Interferon-a (IFN-a) is used to treat hepatitis C. Standard therapy is a weekly injection of PEG-IFN-a , which, while very effective, has debilitating side effects, in particular severe flulike symptoms that occur frequently during the course of the treatment and potentially serious depression. As many patients may be diagnosed but not always feel sick, treatment with PEG-IFN actually makes them feel worse, and there is a high dropout rate. It is worth noting that before the PEGylated version of the molecule, it was injected three times a week, and these side effects were even more pronounced. It has become clear that the flulike side effects are related to the Cmax immediately after injection. We hypothesised that by using an injectable sustained release formulation, if we could reduce the Cmax and sustain blood levels at the minimum therapeutic level (reduce the peak-to-trough ratio), we could reduce side effects and develop a better treatment for patients.
Q Were any studies performed prior to development of the sustained release formulation to check its suitability or establish development targets?
A There was already a considerable amount of clinical data that supported our hypothesis, so we were confident that if we could control burst release and linearity of the release, the resulting lower Cmax would reduce side effects while maintaining pressure on the virus with an effective Cmin. Also, the pharmacokinetics and the target therapeutic plasma levels of the molecule were very well understood, so we focussed on developing a delivery system that could release the protein at the desired rate.
Q What technology was used to produce Locteron? How does it work?
A Locteron is an injectable sustained release microparticle formulation of IFN-a2b that is suspended in an injection vehicle prior to subcutaneous administration and slowly releases the drug. The protein is encapsulated in the polymer microparticles by an emulsion process. The technology that allowed the development of Locteron is PolyActive®—a range of biodegradable poly(ether ester) multiblock copolymers based on poly(ethylene glycol) and poly(butylene terephthalate). Upon administration, the polymers absorb up to 65% of their weight in water and form a hydrogel. Initial release of the protein is controlled by diffusion, which will be accelerated by gradual degradation of the matrix. Since the hydrodynamic radius of the protein is known, the polymer composition is matched with the pore size of the hydrogel so that protein release is controlled. Furthermore, because of this, we can avoid burst release (release of protein in the first few hours after administration)—particularly important for proteins such as IFN-a in which side effects are correlated with Cmax. Furthermore, while emulsion processes have been shown to denature proteins upon encapsulation, the PolyActive polymers self-assemble at the oil–water interfaces, thereby preventing protein degradation on encapsulation. In the case of Locteron, we used in vitro potency assays to demonstrate that IFN-a activity was maintained after encapsulation. Also, we have found there to be an excellent in vitro–in vivo correlation, which has made it possible to predict the pharmacokinetics in man from in vitro dissolution data.
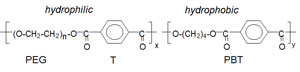 |
| Chemical structure of PolyActive |
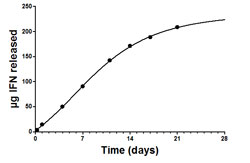 |
| In vitro release of IFN from PolyActive microspheres |
Q How big was the team of scientists that worked on developing the process and formulation? What was its makeup?
A The first project team at OctoPlus consisted of about five scientists, made up of a polymer chemist, formulation scientists, and analysts. Special mention should go to Jeroen Bezemer, who developed the PolyActive-based microspheres, also used in Locteron. The team worked closely in a collaboration with Biolex, who developed and manufactured the IFN-a2b in Locteron in their proprietary Lemna protein expression system. As the product achieved various milestones and advanced through development, the team grew to include process development scientists, quality control and assurance, and regulatory affairs. By the time Locteron went into its first clinical trial, there were about 20 people at OctoPlus working on it, and through phase 2a and 2b trials, there have been at least 20–30 people continuously working on it.
 |
| Transmission electron microscopy photo of a PolyActive microsphere |
Q At what point were scale-up activities begun?
A From the initial laboratory-scale process, the manufacturing process had to be scaled up for nonclinical safety studies and manufacture of clinical trial material. Locteron required a long-term nonclinical safety study, because although the polymer was fairly well established, the combination with IFN-a was novel, and this study eventually ran for two years. The phase 1 clinical trial was a dose ranging study, investigating three different doses, and was produced at a 6 g (polymer mass) batch size. For phase 2, this increased to a 12 g batch size, and we have currently scaled up for phase 3 to a 100 g batch size.
Q Please outline the findings of the clinical studies performed to date on Locteron. What stage in development is it currently at?
A The phase 1 trial in healthy volunteers investigated the safety and pharmacokinetics, and it identified the dose to be used in later trials. This showed that Locteron could be dosed once every two weeks—an improvement on the once a week frequency of the PEGylated molecule—and it was safe and well tolerated. Locteron then went on to be studied in phase 2a and phase 2b studies in chronic hepatitis C genotype 1 patients. The phase 2 studies have shown a 70% reduction in frequency of flulike symptoms and a reduction in the incidence of depression compared with PEGylated IFN. Furthermore, efficacy looks to be marginally improved. We’re very excited by this data, as it looks to be validating our original hypothesis and is a good example of sustained release offering a better treatment for patients.
Q What advice would you give to scientists starting out their career in the controlled release field?
A Make yourself familiar with the business of controlled release products. A good idea is to work backward from an unmet need to ensure you produce something the market wants, not to produce something just because a technology allows you to do so. To be successful, products have to offer something new or at a minimum offer a significant improvement over what is already available out there.
Q Is there a classic paper or an interesting recently published paper you would recommend to the CRS membership?
A Yes. See below.
Recommended Reading
Jorgensen, L, Moeller, EH, van de Weert, M, Nielsen, HM, Frokjaer, S. Preparing and evaluating delivery systems for proteins. Eur. J. Pharm Sci. 29: 174-182 (2006).
De Leede, LGJ, Humphries, JE, Bechet, AC, Van Hoogdalem, EJ, Verrijk, R, Spencer, DG. Novel controlled-release Lemna-derived IFN-a2b (Locteron): Pharmacokinetics, pharmacodynamics, and tolerability in a phase I clinical trial. J. Interferon Cytokine Res. 28: 113-122 (2008).
Dzyublyk, I, Yegorova, T, Moroz, L, Popovych, O, Zaytsev, I, Miroshnichenko, V, Kromminga, A, Wilkes, MM, van Hoogdalem, EJ, Humphries, JE. Controlled release recombinant human interferon-a2b for treating patients with chronic hepatitis C genotype 1: A phase 2a clinical trial. J. Viral Hepatitis 18(4): 271-279 (2011).
Relevant Links
http://www.octoplus.nl/index.cfm/octoplus/products/locteron/index.cfm
© 2012


