E-mails: d.lamprou@qub.ac.uk & e.larraneta@qub.ac.uk
School of Pharmacy, Queen’s University Belfast, 97 Lisburn Road, Belfast, BT9 7BL, UK
Introduction
New materials and manufacturing techniques are emerging with potential to address the challenges associated with the manufacture of pharmaceutical systems that will teach new tricks to old drugs and on the development of new personalised medical devices. Current medical developments are aiming to achieve personalised medicine adapted to patient’s needs. The field of medical devices is following this trend by using manufacturing techniques that allow simple on-demand design/manufacturing of the required device. Techniques such as 3D printing (3DP) have experienced a significant price reduction making possible its use in hospitals for multiple applications. Other techniques such as electrospinning (ES) or microfluidics (MF) have the potential to be used for the preparation of medical devices or drug delivery systems adapted to the requirements of each patient.
3D Printing & Bioprinting
Despite of being extensively used in other industries, 3DP is relatively new to pharmaceutical and medical device manufacturing. 3DP or additive manufacturing (AM) includes a variety of techniques that are able to produce physical models by adding materials layer-by-layer based on a computer-generated design. These designs can present a high degree of complexity while being produced in a reproducible way. 3DP has been used as a rapid and cost-effective technique in wide range of fields, and the term involves many 3D printing processes, which use different types of printing technologies, hundreds of materials, various resolutions and speeds.
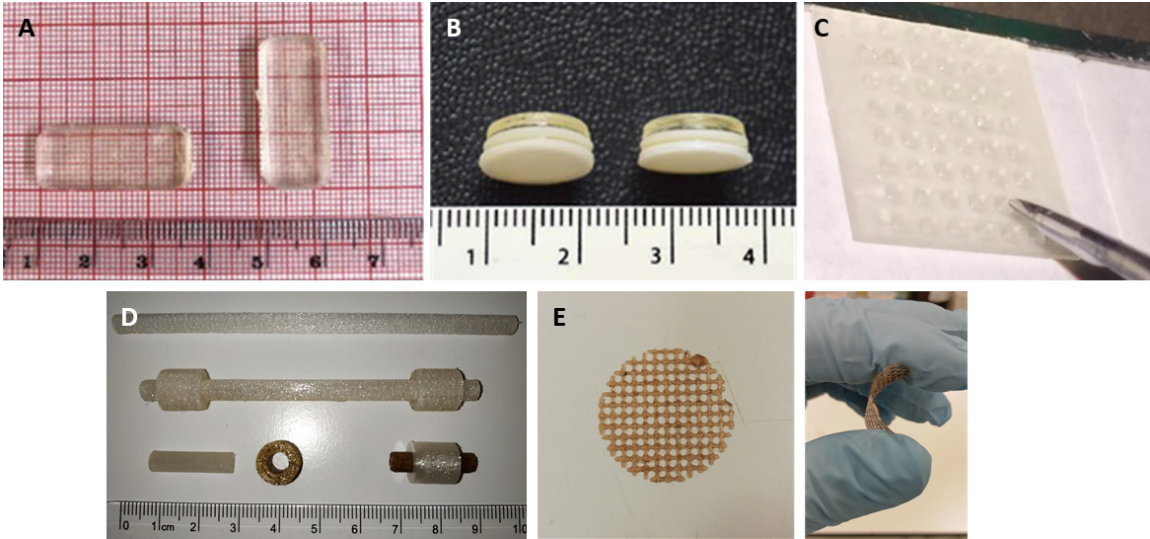
Figure 1. Examples of pharmaceutical and medical devices produced using 3D printing: Oral tablet prepared using SLA (A),1 Oral tablet containing multiple drugs prepared using SLA (B),2 Microneedle array prepared using SLA (C; Reproduced with permission),3 Dyalisis catheters prepared using FDM (D; Reproduced with permission)4 and meshes for wound dressing prepared using FDM (E).
The 3DP techniques, which can be used in pharmaceutical field includes Stereolithography (SLA), Selective Laser Sintering (SLS), inkjet-based 3DP, extrusion based Fused Deposition Modeling (FDM) and pressure-assisted microsyringe (PAM) printing. Our 3D printing facility accommodates over ten 3D printers covering all available types in the market (e.g. FDM, SLS, SLA, Laser, Food), and 4 Hot-Melt Extruders (HME) for the preparation of filaments for the FDM 3DP.
The use of 3DP to manufacture solid oral dosage forms has been extensively reported in the last 5 years (Figures 1A&B). In August 2015 the first-ever 3D-printed drug product, was approved by the FDA (Spritam® - Aprecia Pharmaceuticals). However, there is potential for 3DP beyond the production of conventional oral dosage forms such as medical devices (Figures 1C-E). The technique can be used to prepare patient-matched devices specific to their anatomy using the data obtained from Nuclear magnetic resonance (NMR) imaging or Computer tomography (CT). Examples of devices produced using 3DP includes implants, cardiac valves or even complex drug delivery systems such as microneedles5. Moreover, drugs or other biologically active molecules can be added to the materials used for 3DP. Some examples include antioxidant and anti-infective agents that can be incorporated within the resulting device.6 Anti-infective devices produced using 3DP includes dialysis catheters4 and surgical meshes.
3D Bioprinting, includes all the different modes (e.g. laser-, extrusion-, droplet-), and is been used in different pharmaceutical studies, such as in vitro predictive toxicology, high-throughput screening, drug delivery, in regenerative medicine and tissue engineering. 3D bioprinting is an alternative manufacturing technique that uses micro-extrusion printing. This technique builds objects using extrusion where the biomaterial (bioink) contained in a cartridge is added into the object trough nozzles or needles. For this purpose, bioinks are fluids containing cells. In order to form solid objects the inks need to be able to gelate or to solidify using shear thinning mechanisms or just chemical reactions such as photo-crosslinking. There is a wide variety of bioinks in the market and under development using synthetic (e.g. methacrylates) or natural polymers (e.g. cellulose). These biopolymers will provide mechanical support to cells allowing them to grow within the material. Using this technology, researchers are capable of producing scaffolds to be implanted into a patient for tissue regeneration. In some ways, this technology can be even used to manufacture artificial organs.7 Therefore, this technology presents promising advantages for tissue engineering and regenerative medicine. Currently, within our facilities we have three bioprinters.
Electrospinning & Electrospraying
Several promising techniques have been developed to overcome the poor solubility and/or membrane permeability properties of new drug candidates. Electrospinning & Electrospraying are techniques that utilises electric forces to create Nano- and micro-fibres or micro-particles and co-crystals. Have been widely researched within the scientific field, and in particular within the creation of drug delivery systems (DDSs). The process fundamentally involves applying electric charges across a metallic needle that contains a polymeric solution. When a droplet is formed at the tip of the needle, the induction of charges causes instability – however, there is also a repulsion of charge happening that will overcome the surface tension, which results in the solution accelerating in the direction of the electric field, towards a metallic collector (or target). Currently, a number of different applications are being researched and tested, such as for hernia repair (Figure 2). The type of drugs being studied range from levofloxacin (a type of antibiotic, typically used for treating urinary tract infection, respiratory tract infections, meningitis)8 to dexamethasone (a form of steroid, typically used as anti-inflammatory or to counteract potential side effects in antitumor treatments). A number of DDSs are being developed using these types of drugs, specifically as drug implants.9 Drug implants are very useful DDSs, since can be implanted at the required tissue site, meaning any sustained released of the drug will be high in efficacy (it will achieve the desired therapeutic effect), low in toxicity and reduces the chance of unwanted reactions with irrelevant organs or tissue. The manufacturing of nanofibers for a variety of drug delivery and tissue engineering applications includes oral films, mesh implants, and scaffolds for wound healing. Our facility hosts four electrospinning systems, 3 solutions (can be used also as electrospraying) and one melt.
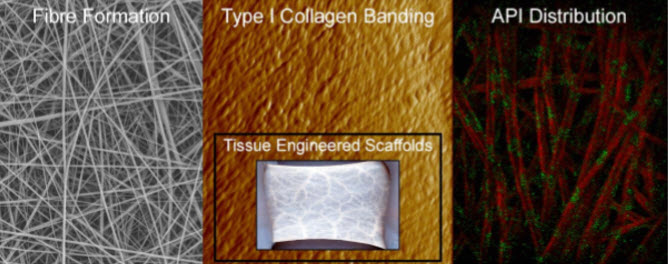
Figure 2. Collagen nanofibers prepared using electrospinning.8
Microfluidics
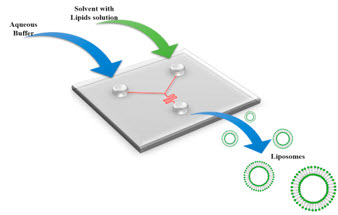
Figure 3. Schematic for a microfluidic device. Reproduced with permission.10
In the last two decades, Nanomedicines (NMs) are being explored for their potentials in treatment of numerous diseases and especially for cancer. Microfluidics is a technique, which deals with flow of fluids within micron-sized channels (Figure 3). It provides a platform where these NMs can be synthesised in a controlled manner enabling to tune their size, charge, polydispersity, and other surface fictionalisation properties. In addition, the technique is energetically economical, easier to use, comparatively cheaper and faster, and the molecules that has not been incorporated in the particles can be reused. Microfluidics have been employed for the formulation of inorganic and polymeric nanoparticles and liposomes10 with precise control over size, Polydispersity index (PDI), and charge, which are critical for the success of the nanoformulations in clinical translation research. It also enables rapid, reproducible and scalable formulation of nanoparticles along with high drug loading. Moreover, can be used for the encapsulation of contrast agents for imaging applications “hiding” the toxicity of the molecules used.11 The microfluidics market includes drug discovery, drug delivery, in-vitro diagnostics, inkjet printing, chemical analysis, and high-throughput screening. Our microfluidic facility hosts five microfluidic devices, including temperature controllers and high-speed microscope for the evaluation of the particles and flows in the microfluidic chips, including the manufacturing of chips.
Conclusions
By using new technologies we can now produce a medical device, which is easier to be operated, have the same (and better) mechanical properties than existed systems, safer, and is loaded with antibacterial agents for a sustained/controlled release. Moreover, personalised medical devices or drug delivery systems are achievable using emerging technologies like 3DP, ES, MF.
Acknowledgments
Research Visitors, Ph.D. Students, and Postdocs in our state-of-the-art facilities.
References
- A.V. Healy , E. Fuenmayor, P. Doran, L.M. Geever, C.L. Higginbotham, J.G. Lyons. (2019) Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics, 11(12), 645.
- P. Robles-Martinez, X. Xu, S.J. Trenfield, A. Awad, A. Goyanes, R. Telford, A.W. Basit, S. Gaisford (2019) 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11(6), 274.
- C.P. Pissinato Pere, S.N. Economidou, G.S. Lall, C. Ziraud, J.S. Boateng, B.D. Alexander, D.A. Lamprou, D. Douroumis. (2018) 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 544(2), 425-432.
- E. Mathew, J. Domínguez-Robles, S.A. Stewart, E. Mancuso, K. O’Donnell, E. Larrañeta, D.A. Lamprou. (2019) Fused Deposition Modeling as an Effective Tool for Anti-Infective Dialysis Catheter Fabrication. ACS Biomater. Sci. Eng. 5(11), 6300-6310.
- S.N. Economidou, C.P. Pissinato Pere, A. Reid, Md. J. Uddin, J.F.C. Windmill, D.A. Lamprou, D. Douroumis. (2019) 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 102, 743-755.
- J. Domínguez-Robles, N.K. Martin, M.L. Fong, S.A. Stewart, N.J. Irwin, M.I. Rial-Hermida, R.F. Donnelly, E. Larrañeta (2019) Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11(4), 165.
- S.J. Lee, J.B. Lee, Young-Woo Park, D.Y. Lee. (2019) 3D Bioprinting for Artificial Pancreas Organ. In: Noh I. (eds) Biomimetic Medical Materials. Advances in Experimental Medicine and Biology, vol 1064. Springer, Singapore.
- I. Hall Barrientos, E. Paladino, P. Szabó, S. Brozio, P.J. Hall, C.I. Oseghale, M.K. Passarelli, S.J. Moug, R.A. Black, C.G. Wilson, R. Zelkó, D.A. Lamprou. (2017) Electrospun collagen-based nanofibres: a sustainable material for improved antibiotic utilisation for tissue engineering applications. Int. J. Pharm. 531(1), 67-79.
- I.J. Hall Barrientos, G.R. MacKenzie, C.G. Wilson, D.A. Lamprou, P. Coats. (2019) Biological performance of Electrospun polymer fibres. Materials. 12(3), 363.
- M. Guimarães Sá Correia, M-L. Briuglia, F. Niosi, D.A. Lamprou. (2017) Microfluidic manufacturing of phospholipid nanoparticles: stability, encapsulation efficacy, and drug release. Int. J. Pharm. 516(1-2), 91-99.
- A. Delama, M.I. Teixeira, R. Dorati, I. Genta, B. Conti, D.A. Lamprou. (2019) Microfluidic encapsulation method to produce stable liposomes containing Iohexol. J. Drug Deliv. Sci. Technol. 54, 101340.


