Introduction
Nowadays, most of the currently available ocular drugs are administered topically through eye drops,1 but their residence time in the eye is short, about 2 min. Only 1–7% of the dose delivered from an eye drop is absorbed by the eye, leading to a final poor drug bioavailability and in some instances to side effects.2
In the last few years, efforts have been made in the attempt to develop new and more efficient ophthalmic drug delivery systems. Among other vehicles, therapeutic soft contact lenses have been demonstrated to be an ideal platform for the controlled delivery of numerous drugs as well as molecules that provide eye comfort.3
To increase drug release duration, Chauhan and coworkers have explored the effect of incorporating vitamin E in contact lenses to create diffusion barriers that can lead to extended release of several ophthalmic drugs.4,5 The hydrophobic and poorly aqueous soluble vitamin E precipitates at the interphase of the biphasic silicone material and acts as a physical chemical barrier to drug diffusion inside the hydrogel.
Bacterial keratitis is a common affliction of the eye, and it is commonly treated by frequent instillation of eye drops. The broad spectrum antibiotic levofloxacin (LVF) (e.g., QUIXIN®, concentration 5 mg/mL) is currently one of the most used drugs for the therapy and prophylaxis of eye infections caused by Staphylococcus aureus and Pseudomona aeuriginosa.
In the present work, the release of LVF from the commercial silicone-based contact lens ACUVUE® TrueEye, preloaded with vitamin E, was investigated. To confirm their adequacy to the ophthalmologic use, the lenses were characterized with respect to their transmittance, wettability, and ionic permeability.
 Experimental Methods
Experimental Methods
Vitamin E and drug loading steps are detailed in Figure 1. ACUVUE® TrueEye lenses were soaked in vitamin E solution dissolved in ethanol (42 mg/mL) for 3 h. Lenses were withdrawn from the solutions, gently blotted, and dried overnight in air, obtaining a vitamin E final w/w fraction of 20%. For the drug loading step, LVF (28266, Sigma Aldrich) was dissolved in phosphate-buffered saline (PBS) (5 mg/mL). Vitamin E loaded lenses were soaked in this solution for 7 days. For the control, the vitamin E loading step was skipped.
Drug release experiments from control and vitamin E loaded contact lenses were performed in sink conditions in 3 mL of PBS, at room temperature. At predetermined time intervals, the absorbance spectra of the supernatant solution were measured over the wavelength range of 265–305 nm using a UV-visible spectrophotometer (Thermospectronic Genesys 10 UV).
The ion permeability of the lenses was assessed by measuring the release profiles of salt in sink conditions (n = 3). Lenses were previously soaked in 0.75M NaCl solution overnight to load the salt. The release was done into 36 mL of well-stirred (300 rpm) deionized water. The NaCl concentration of the aqueous medium was monitored using a Con 110 series sensor (OAKTON®).
Optical clarity studies were carried out measuring the transmittance of the lenses with a UV-visible Beckmam DU-70 spectrophotometer. Three independent experiments were done.
 The wettability of the hydrated contact lenses was characterized by measuring the water contact angles through the captive bubble method. Bubble images were acquired using a video camera ( JAI CV-A50) attached to a microscope (Wild M3Z), which was
The wettability of the hydrated contact lenses was characterized by measuring the water contact angles through the captive bubble method. Bubble images were acquired using a video camera ( JAI CV-A50) attached to a microscope (Wild M3Z), which was
connected to a frame grabber (Data Translation DT3155). The image analysis was performed with ADSA-P software (axisymmetric drop shape analysis profile).
Results and Discussion
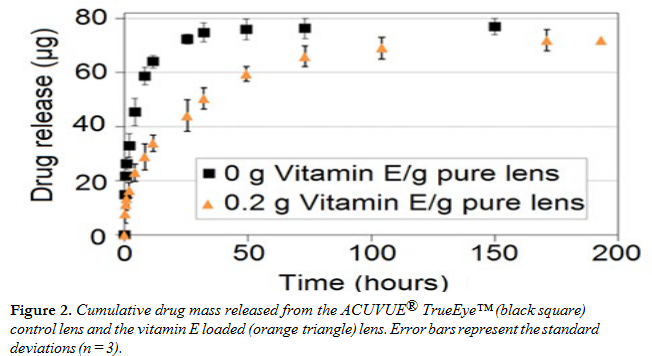
Figure 2 shows LVF release profiles in fresh PBS from ACUVUE® TrueEye™ lenses with and without vitamin E. The lenses released 90% of the loaded drug in 32 h. With the inclusion of 20% vitamin E, the drug release duration (for 90% of the total mass released) increased to 100 h, exhibiting a threefold increase of the release time. The mass of drug released was not impacted by vitamin E incorporation. The lenses were subjected to a second cycle of release to test the validity of the sink assumption. The data showed negligible release of LVF in the second release cycle, proving that the release conditions for the first cycle can be considered as sink, and that the total amount of drug loaded was released.
The ion permeability of the control lenses was 1.1 × 10–7 ± 0.6 × 10–7 cm2/s (n = 3), and it decreased to 0.7 × 10–7 ± 0.4 × 10–7 cm2/s (n = 3) in the presence of vitamin E. In both cases the ion permeability was above the minimum acceptable value (2.5 × 10–8 cm2/s).6
 The presence of vitamin E caused a decrease in the transmittance by about 5%, but both samples (with and without vitamin E) presented values of transparency over 90%, matching the transmittance characteristics of soft contact lenses.
The presence of vitamin E caused a decrease in the transmittance by about 5%, but both samples (with and without vitamin E) presented values of transparency over 90%, matching the transmittance characteristics of soft contact lenses.
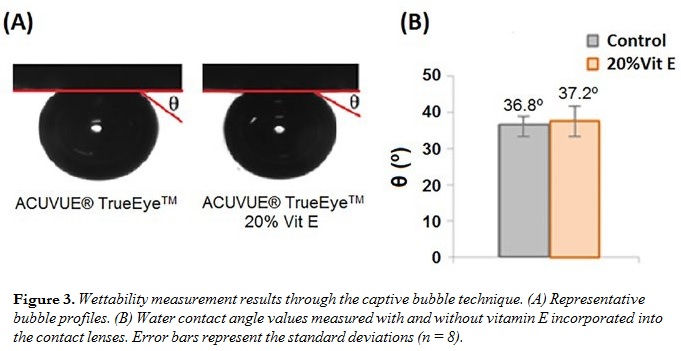
The contact angle results (Figure 3) showed that the presence of vitamin E did not significantly alter the wettability.
Conclusion
Vitamin E loading in commercial silicone contact lenses can evidently increase the release duration of LVF antibiotic, without compromising transparency, wettability, and ionic permeability. The increase in duration happens owing to the presence of the vitamin E nanoaggregates formed into the lenses, which have a barrier effect for the drug release. The drug release duration achieved shows that a contact lens would cover the most acute phase of bacterial keratitis, avoiding the annoying instillation of eye drops during the first 4 days. For a detailed treatment of this topic, see our upcoming article “Controlled Release of Antibiotics from Vitamin E–Loaded Silicone-Hydrogel Contact Lenses” in Journal of Pharmaceutical Sciences.
Acknowledgements
To Fundação para a Ciência e a Tecnologia for Patrizia Paradiso’s Ph.D. grant (SFRH/BD/71990/2010) and for the funding through the projects UID/QUI/00100/2013 and M-ERA.NET/0005/2012.
aCentro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, 1049-001, Portugal.
bCorresponding author. E-mail: patrizia.paradiso@tecnico.ulisboa.pt
cCiiEM, Instituto Superior de Ciências da Saúde Egas Moniz, Monte de Caparica, Caparica, 2829-511, Portugal.
dDepartment of Chemical Engineering, University of Florida, Gainesville, FL 32611-6005, U.S.A.
References
- Lang, JC. Ocular drug delivery conventional ocular formulations, Adv. Drug Deliv. Rev. 16:39-43 (1995).
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics, Adv. Drug Deliv. Rev. 58:1131-1135 (2006).
- White, C, Byrne, M. Molecularly imprinted therapeutics contact lenses, Expert Opin. Drug Deliv. 7(6):765-780 (2010).
- Peng, CC, Chauhan, A. Extended cyclosporine delivery by silicone-hydrogel contact lenses, J. Controlled Release 154(3):267-274 (2011).
- Hsu, KH, Fentzke, RC, Chauhan, A. Feasibility of corneal drug delivery of cysteamine using vitamin E modified silicone hydrogel contact lenses, Eur. J. Pharm. Biopharm. 85(3 Pt A):531-540 (2013).
- Nicolson, PC, Baron, RC, Chabrecek, P, Court, J, Domschke, A, Griesser, HJ, Ho, A, Hopken, J, Laycock, BG, Liu, Q. Extended wear ophthalmic lens. Patent number US5760100 A (1998). n


