Drugs and medical devices represent two modalities in treating disease, which display some fundamental differences. Drug molecules, when administered by conventional means, rarely remain in the body at therapeutic levels for extended time periods. Implantable devices such as pacemakers and vascular grafts, on the other hand, are usually designed to persist within the body and maintain their function for extended periods of time. (Exceptions to the rule occur for implanted devices that are meant for short-term purposes. Such devices can be retrieved later or can be programmed for degradation and resorption.)
Devices have long been used to improve drug therapy. Tablets, capsules, oral liquid syringes, eye drops, nose drops, syringes for intravenous, subcutaneous, or intramuscular injection, and catheters are classical drug delivery devices. While these devices continue to be perfected, the last 50 years has seen the introduction of numerous advanced drug delivery devices that are designed to improve the timing and placement of drug administration. The motivation behind such spatiotemporal control has been due, in part, to improved understanding of the roles of pharmacokinetics (ADME), mass transport through biological barriers, receptor-mediated pharmacodynamics, and toxicology in determining how a drug should best be administered. Members of CRS need only think of external or implantable electromechanical pumps, osmotic pumps, passive- and electrotransport-driven skin patches and microneedle arrays, ocular inserts, and specially designed intranasal and pulmonary applicators (which control the location in the nasal cavity or lung in which particles are deposited) as examples in which mechanical, chemical, and electrical devices can improve drug delivery. Other devices include injectable micro- and nanoparticles, whose physical (size, porosity) and chemical (targeting ligands, degradation) properties are designed in to optimize the spatial and temporal distribution of the active agent.
The previous paragraph emphasizes the use of devices to improve drug therapy. Conversely, how can drugs enhance device therapy? Insertion of any device involves some insult to the local tissues and may result in infection due to incomplete sterility of the procedure, local inflammation, the inevitable foreign body reaction, or a systemic immune response. Traditionally, this challenge has been met by administering antibiotics, anti-inflammatories, and immune response modifiers before, during, or after the procedure. Well-known examples include oral penicillin used as an adjunct to oral surgery (often involving installation of a periodontal device) in order to prevent bacterial colonization on susceptible heart valves, and prescription of Plavix to prevent inflammation following surgical placement of a coronary stent. Along similar lines, transcutaneous catheters are notoriously susceptible to infection, and much interest has been shown in coating the lumens of such catheters with antibiotics that elute throughout the period of catherization.
Systemic administration of drugs following a surgical or local incision procedure can treat acute reactions without significant danger to the patient. However, chronically implanted devices may require more sustained delivery to ameliorate local reactions, and long-term systemic delivery of pharmacologic countermeasures is out of the question due to issues of convenience, compliance, and eventual onset of toxicity as drug accumulates throughout the body. For this reason, considerable effort has been expended in developing drug/ device combination products, especially for chronic conditions. In the next few paragraphs, we provide examples.
Cardiac rhythm control, or pacemaking, has been one of the most significant developments in modern medicine and has saved millions of lives. While early pacemakers were bulky and required transcutaneous delivery of current from an external power source to the implanted pacemaker lead, advances in microprocessing and battery technology have permitted total implantation. 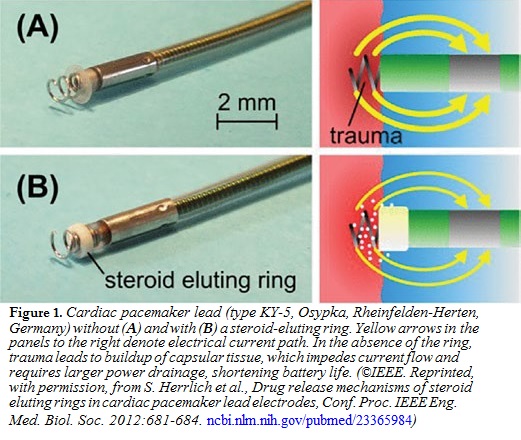 The pacemaker lead is placed directly into the myocardium, where it delivers pacing current pulses. After placement of the lead, however, a fibrous capsule forms around it, increasing the resistance of the lead-myocardial interface. By Ohm’s law, voltage = current × resistance, the voltage required to maintain the required current
The pacemaker lead is placed directly into the myocardium, where it delivers pacing current pulses. After placement of the lead, however, a fibrous capsule forms around it, increasing the resistance of the lead-myocardial interface. By Ohm’s law, voltage = current × resistance, the voltage required to maintain the required current
must increase. According to the power relation, power = voltage × current, the amount of power per pulse also increases, and power is drained from the battery more rapidly, shortening the battery’s lifetime. Recognizing this problem, pacemaker leads are regularly accompanied by small cuffs, which slowly and steadily release anti-inflammatory steroids such as dexamethasone into the surrounding myocardial tissue (Figure 1). The steroid attenuates formation of the fibrous capsule, keeps electrical resistance and power drainage low, and enhances battery lifetime. Because the steroid is targeted directly to the cardiac tissue, its dosing rate is a tiny fraction of what would be required if the steroid were administered systemically, and toxic side effects are negligible.
A second class of devices that have benefited from local drug release are coronary stents. Following a heart attack, the enzyme streptokinase is injected into the thrombotic coronary artery, dissolving the clot and permitting reestablishment of blood flow. Then, a stent consisting of a metal mesh, surrounding an inflatable balloon, is threaded into the coronary artery at the tip of a guiding catheter under angiographic visualization. Once properly situated, the balloon is inflated and the stent ratchets into place, keeping the artery open. The catheter and balloon are then withdrawn. The earliest coronary stents were made of bare, polished stainless steel. While these “bare metal” stents were successful in maintaining structural integrity of the arterial walls, regrowth of arterial smooth muscle and epithelium into the arterial lumen, or restenosis, occurred in about 25% of the surgical patients. To alleviate this problem, various anti- inflammatory (e.g., sirolimus) and anti-proliferative (e.g., paclitaxel) drugs are sprayed onto the stent surfaces in conjunction with a 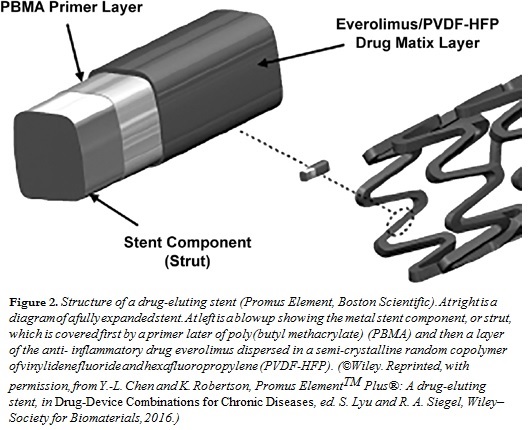 polymer, which controls the rate of release of the drug into the surrounding arterial tissues over a prescribed time period, thus attenuating inflammatory and/or neoproliferative processes. Such drug-eluting stents (Figure 2) are masterpieces of engineering, because they must be designed to release drug in a controlled manner over weeks to months, such that a large fraction of the released drug is available to the local tissue, and such that the coating film does not detach from the underlying metal. As with the steroid-eluting lead, it is possible to achieve high local concentrations of the drug while avoiding significant buildup of drug levels elsewhere in the body. Drug-eluting stents have been shown to reduce the restenosis rate to about 5%.
polymer, which controls the rate of release of the drug into the surrounding arterial tissues over a prescribed time period, thus attenuating inflammatory and/or neoproliferative processes. Such drug-eluting stents (Figure 2) are masterpieces of engineering, because they must be designed to release drug in a controlled manner over weeks to months, such that a large fraction of the released drug is available to the local tissue, and such that the coating film does not detach from the underlying metal. As with the steroid-eluting lead, it is possible to achieve high local concentrations of the drug while avoiding significant buildup of drug levels elsewhere in the body. Drug-eluting stents have been shown to reduce the restenosis rate to about 5%.
While we have focused primarily on cardiac applications, local drug delivery may serve to increase the patency and efficacy of other devices used to treat a variety of ta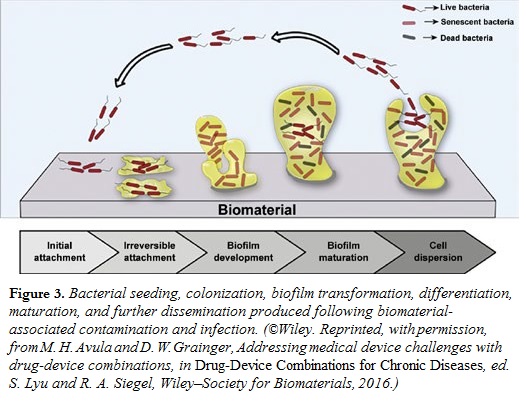 rget conditions. Another important application is to maintain a microbe-free environment surrounding an implanted device. Any incision poses risk of microbial infection, and bacteria are notoriously effective in forming colonies, or biofilms, on surfaces. Following colonization, bacteria go into a dormant state, but some unpredicted triggering event, which may happen years later, “wakens” the bacteria, which then detach and invade the blood stream. Bacteria in biofilms are highly resistant to antibiotic therapy and require much higher doses compared with circulating, planktonic bacteria (Figure 3). Incorporation of antibiotics near or on the surfaces of medical devices may prevent them from being colonized.
rget conditions. Another important application is to maintain a microbe-free environment surrounding an implanted device. Any incision poses risk of microbial infection, and bacteria are notoriously effective in forming colonies, or biofilms, on surfaces. Following colonization, bacteria go into a dormant state, but some unpredicted triggering event, which may happen years later, “wakens” the bacteria, which then detach and invade the blood stream. Bacteria in biofilms are highly resistant to antibiotic therapy and require much higher doses compared with circulating, planktonic bacteria (Figure 3). Incorporation of antibiotics near or on the surfaces of medical devices may prevent them from being colonized.
While it may seem simple to combine already utilized drugs and devices, experience in the industry has shown that it may take years of development to get it right. Regulatory agencies are also concerned with the compounding of risks whenever a combination therapy is proposed. We see combination drug/device therapies as having great future promise, but much needs to be learned regarding how they can be developed optimally.
Ronald A. Siegel and SuPing Lyu are co-editors of a recent book titled Drug-Device Combinations for Chronic Diseases (Wiley–Society for Biomaterials, 2016). For more information, visit wiley.com/ WileyCDA/WileyTitle/productCd-1118120000.html. n
1Professor of Pharmaceutics and Biomedical Engineering, University of Minnesota, U.S.A.
2Scientist and Technical Fellow, Medtronic, U.S.A.


