The issue of poor water solubility and the role of drug nanocrystals
Poor water solubility of drugs remains to date a major challenge for drug delivery scientists working in academic and industrial labs, since it affects directly drug performance and absorption. In order to increase drug solubility through molecular changes, a wide range of alternatives have been investigated. These include the creation of salts, prodrugs, use of cosolvents, complexation with cyclodextrins, co-crystallization, co-amorphization, solid dispersions, use of metastable polymorphs. Over the last two decades the formulation of nanoparticle-based drug delivery systems, such as lipid-based nanoparticles, have been amongst the most relevant approaches for the delivery of hydrophobic compounds. However, these tactics are often associated with drawbacks, many of which are related to the inherent physicochemical characteristics of the drug molecule, like low drug loading (5–30% w/w), limited long-term stability and in some cases, requirement of organic solvents during manufacture. Particle size reduction is one the most accepted strategies to increase drug dissolution, firstly to the micrometre range (< 10 µm), and in the early 2000’s below 1 µm, giving place to the term nanocrystals (NCs).
NCs are crystalline drug particles with mean sizes in the nanometre range and are generally covered in a stabilising layer. The particles in suspension are known as nanosuspensions, and the solvent can be removed using various methods, including freeze- or spray-drying to produce NCs as re-dispersible powders. NCs have been widely used to deliver hydrophobic drugs, resulting in the approval of more than twenty products and a significant number of clinical studies which are in various stages of development. When compared to alternative nanoparticle-based drug delivery technologies, NCs have numerous advantages. In contrast to polymeric and lipidic nanoparticle drug delivery systems, NCs are free of carrier components and have up to 100% loading capacity. They can be produced without the use of organic solvents, obtained at neutral pH, and have good stability over time. These features collectively make NCs an appealing technology platform for the delivery of hydrophobic medicines via multiple administration routes, including oral, ophthalmic, pulmonary, injectable, mucosal, transdermal, and intranasal, as illustrated in Figure 1.
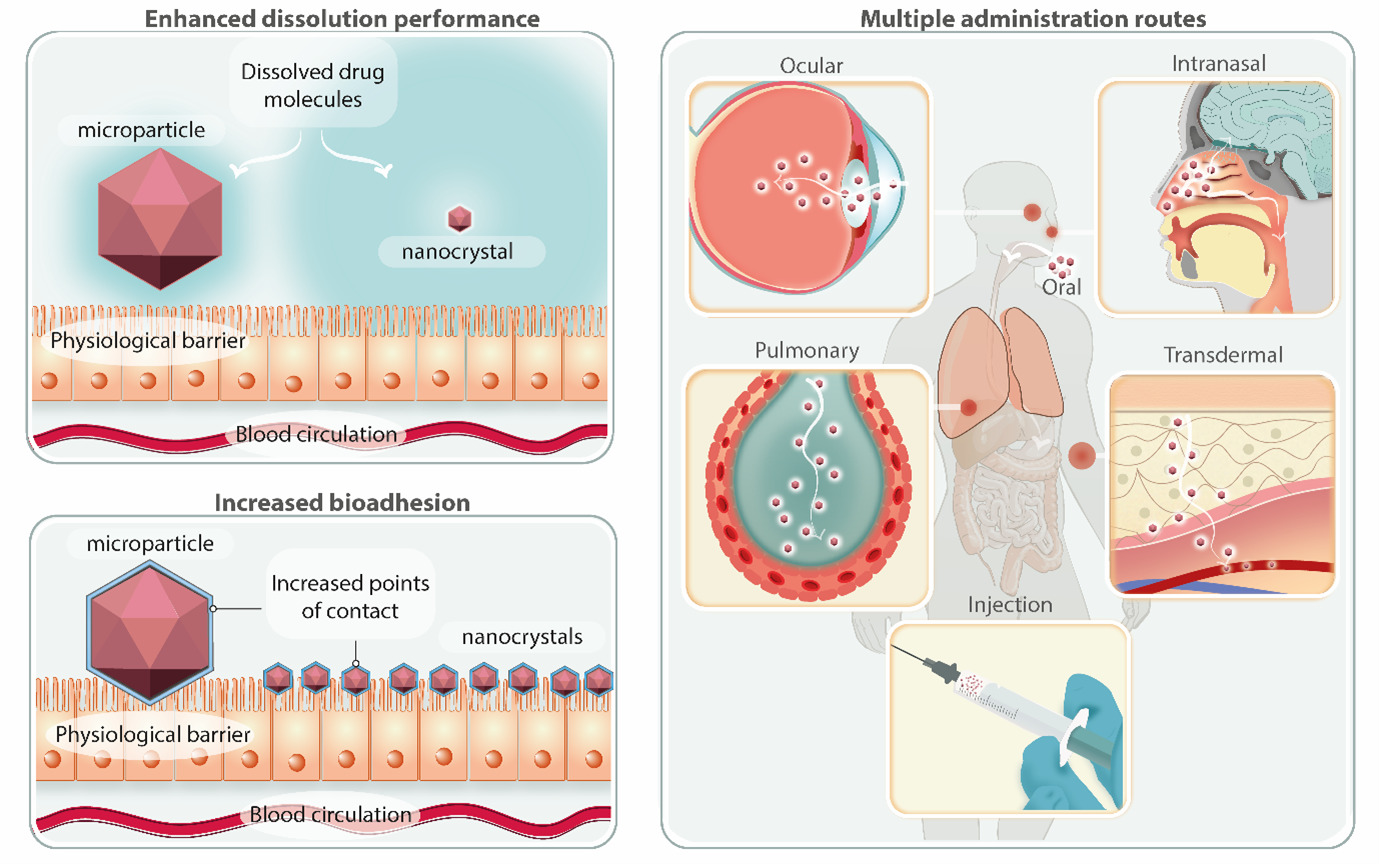
Figure 1. Summary of the key features of nanocrystals, including an increased dissolution rate, saturation concentration, and mucoadhesion (left panels). These features enable the administration of poorly-soluble drugs through multiple administration routes (right panel). Reprinted from Reference 1, with permission from Elsevier, Copyright® 2022.
A recent report on a NCs-based formulation for long-acting drug delivery to mucosal tissues
The delivery of drugs to mucosal tissues remains a substantial challenge due to the physiological characteristics of such tissues, where the secretion of fluids easily removes topical formulations, and drug permeation is impaired by the presence of a mucus layer and enzymatic degradation. Sparking interest in the rational design of novel drug delivery systems to mucosal tissues, our group has recently described an innovative work combining highly elastic sticky eutectogels (Egels) and NCs, showing promise for long-acting drug delivery to the mucosa after topical application. The article, published in Materials Today Bio, reports the manufacture and characterisation of this novel platform and its application to porcine mucosal models. Egels are a new kind of biocompatible ionic material prepared in the absence of water, by heating glycerol and choline hydrochloride together to form deep eutectic solvent, with subsequent addition of gelatin. The synergistic interactions among the Egel components produce a bioadhesive material with exceptional flexibility and stretchability (up to 160%), appropriate attachment to mucin protein (40 kPa) and reversible gel-sol phase transition at 50°C. Interestingly, this rising technology possesses the capability to maintain the integrity of the dispersed NCs after casting and re-casting the gels, as the their particle size and polydispersity index did not differ significantly during the process, as observed in Figure 2. This interesting property, widens the scope of manufacture, enabling 3D printed formulations providing a more tailored approach for the production of personalised medicines. Alteration of the composition of materials used to manufacture the Egels enables manipulation of the viscoelastic and mechanical properties of the material. Such versatility opens the door to explore the multitude of conditions which develop within mucus membranes such as mucosal fungal infections or trichomoniasis by implementation of antimycotic drugs and antibiotics within the Egels, respectively.
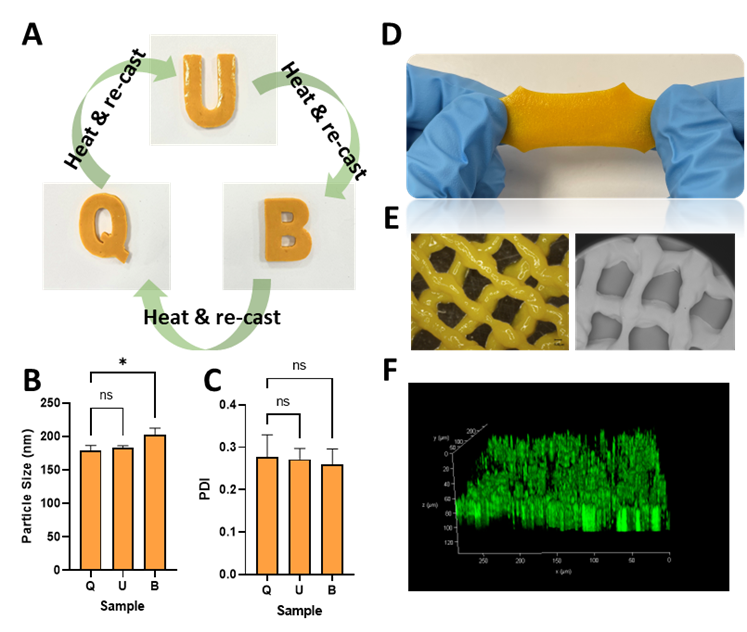
Figure 2. Recently reported eutectogels supporting drug NCs for mucosal drug delivery. A- Reversible casting of the NCs-loaded Egels. B, C- Particle size and PDI of NCs redispersed from the QUB gels. D- The novel Egels are very stretchable. E- Microscopical examination of 3D-printed meshes. F- Multi-photon microscopy of neonatal porcine mucosa treated with Egel-NCs. Adapted from Reference 2, with permission from Elsevier, Copyright® 2022.
References
[1] M.B. McGuckin, J. Wang, R. Ghanma, N. Qin, S.D. Palma, R.F. Donnelly, A.J. Paredes, Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes, J. Control. Release. 345 (2022) 334–353. https://doi.org/https://doi.org/10.1016/j.jconrel.2022.03.012.
[2] M.B. Bianchi, C. Zhang, E. Catlin, G. Sandri, M. Calderón, E. Larrañeta, R.F. Donnelly, M.L. Picchio, A.J. Paredes, Bioadhesive eutectogels supporting drug nanocrystals for long-acting delivery to mucosal tissues, Mater. Today Bio. (2022) 100471. https://doi.org/https://doi.org/10.1016/j.mtbio.2022.100471.


