
Q Thank you for agreeing to speak with us to share some of your experiences during the development of Bydureon (exenatide extended-release for injectable suspension) and to give CRS members an insight into its development. First, though, congratulations! Bydureon is now approved by the U.S. FDA and in various territories outside the United States. What you’ve achieved is no mean feat, and on behalf of the CRS community, we would like to take the opportunity to say well done!
A Thank you. This approval is a significant milestone, as it symbolizes why it’s important to approach science with the patient in mind. Because, at the end of the day, the science disappears at the bedside.
Q What makes exenatide an attractive candidate for an injectable sustained-release formulation?
A For many people, trying to maintain the appropriate blood sugar (glucose) levels day after day can be frustratingly difficult. Other formulations of GLP-1 receptor agonists require twice-daily or once-daily administration. In contrast, Bydureon only needs to be self-administered once a week. For example, following subcutaneous administration, the active ingredient exenatide, which is a GLP-1 receptor agonist, reaches its peak plasma concentration in 2 hours and is subsequently eliminated with a terminal half-life of 2.4 hours. Given this relatively short action, a long-acting formulation of exenatide exhibiting blood glucose control across the fasting and postprandial periods, as well as maintenance of Hb1Ac levels long-term, offers significant clinical utility.
Bydureon consists of exenatide encapsulated in microspheres using Alkermes’s proprietary technology for long-acting medications. As the first and only once-weekly treatment for diabetes, Bydureon provides continuous exposure to exenatide following weekly patient-administered subcutaneous injections. Because of this, patients taking Bydureon have greater ability to meet therapeutic Hb1Ac goals, better fasting glucose control, a lower incidence of gastrointestinal upset, more flexible dosing, and less frequent injections (one versus up to 14 per week).
Q Were any studies performed prior to development of the sustained-release formulation to check its suitability or establish development targets?
A The Bydureon development program was initiated based on successful phase 2 clinical data for Byetta®. Our initial targets were based on the Byetta development program, and we refined targets as we gained more experience with the exenatide molecule in the clinic and commercial settings.
Q What technology was used to produce Bydureon? How does it work?
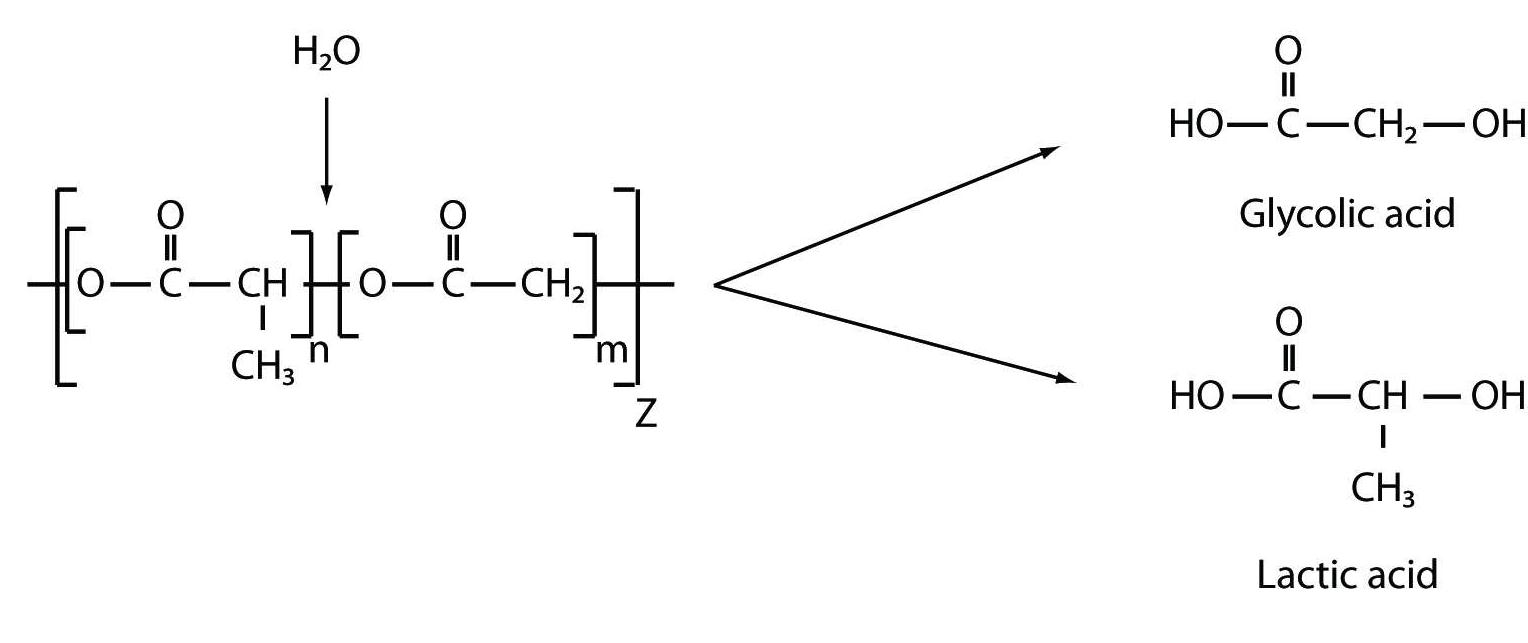
A Bydureon is based on the Medisorb® polymer microsphere technology (Alkermes, Inc., Waltham, MA, U.S.A.). Other pharmaceuticals utilizing this technology include Risperdal® Consta®, an extended-release formulation of the atypical antipsychotic risperidone, which is used to treat schizophrenia and bipolar disorder, and Vivitrol®, an extended-release formulation of naltrexone that is used to treat alcohol and opioid dependence. Both Risperdal Consta and Vivitrol are administered by intramuscular route by trained healthcare professionals. In contrast, Bydureon is administered subcutaneously by patients or their caregivers. In general terms, the proprietary Medisorb technology encapsulates a medication of interest in injectable microspheres, which slowly degrade in situ and release drug into circulation in a sustained fashion. The structural matrix of the microsphere is composed of a medical-grade biodegradable polymer called poly-(D,L-lactide-co-glycolide) (PLG) (Figure 1). These polymers have been used in surgical sutures, bone plates, and orthopedic implants for decades and in microsphere form as a long-acting drug delivery system since 1984. Degradation of the PLG polymer occurs by natural (i.e., non-catalyzed) hydrolysis of the ester linkages into lactic acid and glycolic acid, which are naturally occurring substances that are easily eliminated as carbon dioxide and water.
 |
|
Figure 1: Hydrolysis of PLGA to Glycolic and Lactic acids |
It is important to note that the encapsulated exenatide in Bydureon that is released into circulation is identical to Byetta, meaning that the extended-release mechanism arises solely from the microspheres.
Q How big was the team of scientists that worked on developing the process and formulation? What was its makeup?
A The development team consisted of scientists from three companies working together: Alkermes, Amylin Pharmaceuticals, Inc., and Eli Lilly and Company. Alkermes was responsible for developing the formulation and process leading to commercial manufacturing. On the chemistry, manufacturing, and controls (CMC) side, dozens of staff were working across different sites, in R&D and in clinical and ultimately commercial manufacturing, for a sustained period of almost a decade. So this has truly been a big effort from a sizable team.
Q I’m sure the target product profile contained a number of targets that might have been difficult to achieve simultaneously. How did the development strategy approach this? Could you give us some examples?
A The formulation and process development activities revolved around 1) developing a formulation that provides minimal burst and provides an acceptable peak-to-trough plasma concentration (Cmax to Cave) ratio of less than 3; 2) selecting a polymer that simultaneously provides the required extended duration of release while maintaining the peptide in a stable form on storage and in vivo; and 3) developing a process that is scalable to commercial production.
We used a variety of in vitro selection and screening tools and animal pharmacokinetic models during the development program. Several studies were required to develop the final Bydureon formulation. We initially investigated various excipients that would modulate release and act as stabilizers to maintain peptide integrity during manufacturing. The optimal formulation, which exhibited extended release of exenatide at consistent therapeutic levels over the dosing interval while also providing an acceptably low initial release, contains exenatide and sucrose encapsulated in PLG 50:50 DL 4AP polymer.
Q What factors were considered in selecting the polymer to encapsulate the peptide?
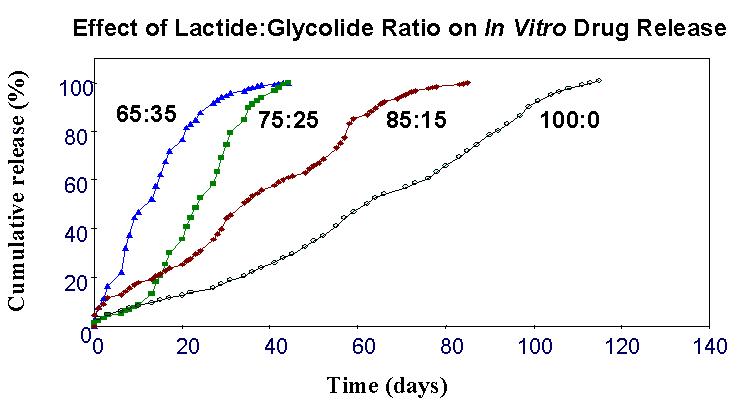
A The duration of the extended release can be altered by choosing the appropriate type of PLG matrix. Increasing the ratio of lactide to glycolide in the matrix, for instance, decreases the overall hydrophilicity of the microspheres and thereby reduces the rate of biodegradation (see Figure 2). Conversely, adding carboxylic acid end groups to the PLG polymers increases the hydrophilicity of the microspheres, favoring faster release. Increments or decrements in polymer molecular weight can reduce or increase, respectively, biodegradation rates. Thus, by choosing the ideal lactide-to-glycolide ratio, functional end group, and molecular weight of the polymer, one can modulate drug release from the PLG matrix. Another important factor, in addition to selecting the polymer, is to ensure the peptide has a stable shelf life and is stable upon formation of the depot in vivo. In the case of Bydureon, this was achieved by adding the excipient sucrose.
 |
Q Peptides and proteins can degrade in PLG during encapsulation and release, although there are a number of published formulation strategies to mitigate this. Did you find this an issue to address during the development of Bydureon? If so, how did you overcome it?
A A number of excipients have been used to stabilize proteins and peptides in formulation, including carbohydrates (sugar) and salts, such as ammonium sulphate, and so on. We studied the stability impact of a number of excipients during development. Sucrose is included in the formulation because it provides stability during processing as well as during storage. Also, the type and amount of excipients were carefully selected to ensure that the initial release, or “burst,” was kept minimal.
Q Burst release from these types of systems is often a difficult issue to control, but the published data on Bydureon show it to be very low. Was this initially an issue for Bydureon? If so, can you give us an insight into how you overcame it?
A One of the adverse effects of the GLP-1 class of drugs is nausea and vomiting. A spike in drug concentration (or burst) will lead to unpleasant nausea in patients taking the GLP-1 drugs. Our development efforts focused on controlling or minimizing the ratio of burst plasma concentration to the average plasma concentration (Cmax/Cave) through the duration of release. We devoted a significant portion of development time to understanding the factors leading to burst, so we could fine-tune the formulation and process to achieve the desired release profile.
Q Did you apply the principles of Quality by Design during the development of Bydureon? If so, at what stage did you start?
A We used sound statistical design of experiments to identify the process design space during scale-up and process optimization. We did most process optimization and process understanding experiments at the “intermediate” scale. We used the learnings from the intermediate scale to develop an efficient and robust process at the commercial scale.
Although Quality by Design guidance was not published when this project started, significant development work was performed to assure deep process understanding. As Q8 was drafted and revised, we tried to incorporate those principles into our development. We also utilized process analytical technology (PAT) during development in the form of Lasentec® on-line particle monitoring during coacervation and on-line mass spectrometry during drying.
Q At what point were scale-up activities begun?
A We used a laboratory-scale process during the proof of concept stage and intermediate- and commercial-scale ones during phase 3 clinical trials. Alkermes developed the process and scale-up and transferred the process successfully to the Amylin-owned commercial manufacturing facility.
Q What were the major challenges you faced during the development of Bydureon?
A Technical challenges included identifying and optimizing the formulation and process to get the desired Cmax/Cave ratio. The manufacture of Bydureon involves a complex coacervation process (also known as phase separation). Very limited literature on the coacervation process is available to the public, and we were the first to develop a multi-kilogram-scale commercial coacervation process for a parenteral drug product. It was quite a challenge not only to develop a robust process but also to make it suitable for state-of-the-art sterile manufacturing.
Another key challenge was the development of a suitable in vitro release test method, as in vitrorelease is a key quality attribute to evaluate and demonstrate acceptable product performance. Unlike the situation for oral formulations, there are no standardized test procedures (e.g., USP methods) for parenteral microsphere products, and the process of establishing acceptance criteria is more complicated. In addition, we developed the in vitro release methods to be biorelevant in that they mimic the duration and release patterns seen in vivo in appropriate animal models.
Q What advice would you give to scientists starting out their careers in the controlled release field?
A I would like to borrow a passage from Melvin Calvin’s autobiography, Following the Trail of Light: A Scientific Odyssey (1992): “The synthesis of a really new concept requires some sort of union in one mind of the pertinent aspects of several disciplines…. It’s no trick to get the right answer when you have all the data. The real creative trick is to get the right answer when you have only half of the data in hand and half of it is wrong and you don’t know which half is wrong. When you get the right answer under these circumstances, you are doing something creative.” It is really important to have a team that can practice risk-based decision making on the basis of good science and engineering and can make decisions that take account of the fact that we cannot have all the information we would like to make any given decision in a fast-paced development program.
Q Is there a classic paper or an interesting recently published paper you would recommend to the CRS membership?
A For those interested in learning more about Bydureon’s development, I recommend reading “Encapsulation of Exenatide in Poly-(D,L-lactide-co-glycolide) Microspheres Produced an Investigational Long-Acting Once-Weekly Formulation for Type 2 Diabetes,” authored by DeYoung et al.
If you are developing a microsphere or controlled release drug product, the following article may be useful: “Points to Consider When Establishing Drug Product Specifications for Parenteral Microspheres,” authored by Kumar and Palmieri.
References
DeYoung, MB, MacConell, L, Sarin, V, Trautmann, M, Herbert, P. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes, Diabetes Technol. Ther. 13(11): 1145-1154 (2011).
Kumar, R, Palmieri, MJ. Points to consider when establishing drug product specifications for parenteral microspheres, AAPS J. 12(1): 27-32 (2010).
Relevant Links


