by Patricia Comeau, Dalhousie University, Nova Scotia

Dr. Sylvain Martel
Interview with Dr. Sylvain Martel, Department of Electrical and Computer Engineering, Institute of Biomedical Engineering, and NanoRobotics Laboratory, Ecole Polytechnique de Montreal about his recent article in Biomaterials.
Background
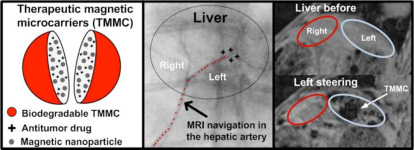
Thirty years ago, magnetic tumor targeting was proposed to increase the concentration of the cytotoxic agent in the tumor. However, despite some improvements in targeting efficiency, this approach still delivers a significant number of the targeting carriers to healthy tissues and organs after systemic administration. In addition, this targeting is only applicable for superficial cancers and is not capable of targeting deep tissues efficiently. A new approach, namely, magnetic resonance navigation (MRN), has been proposed to overcome the limitations of the older systems. It has shown great potential in the targeted delivery of endovascular magnetic carriers in deep tissues to areas of interest, while minimizing systemic carrier distribution. In this paper, Dr. Martel and co-authors reported on a proof-of-concept preclinical study in which they considered the preparation and steering of therapeutic magnetic microcarriers (TMMCs) designed according to MRN and chemoembolization constraints. Figure 1 depicts their TMMC targeting in use in vivo.
|
|
| Figure 1. Targeting of TMMC in the left lob of a liver of a rabbit using an upgraded clinical MRI scanner. |
Q Please explain the design requirements for the TMMC and how these will be monitored prior to use in a clinical setting.
A Simply speaking, there are three components that must be integrated for the implementation of TMMC, namely, the magnetic nanoparticles, the therapeutic load, and finally, a biodegradable material capable of encapsulating both. The magnetic nanoparticles have two functions. First, they act as MRI contrast agents, allowing us to track the displacement of the TMMC and to assess the amount of TMMC and, therefore, the amount of therapeutic agents that reached the targeted region. Second, the same magnetic nanoparticles allow for the propulsion and steering of the TMMC along a path in the blood vessels and toward the final destination. Made of soft magnetic material, they become fully magnetized once in a high homogenous magnetic field, such as when operating inside a clinical MRI scanner. By superposing 3D magnetic gradients (similar to the ones used in MRI to select an image slice of the patient) on top of this high homogenous field, we can navigate the TMMC precisely along a predefined path using the nanoparticles acting like “nanomotors,” where the directional magnetic gradients induce a propelling force on the same nanoparticles. Since the propelling force depends on the total amount of magnetic material in each TMMC, we must have sufficient nanoparticles for effective navigation while providing space to load the therapeutic agent. As an example, approximately 30–40% for 50 micron TMMC is typically used. Finally, the envelope or body of the TMMC can be made of various materials. We already experimented with various types of materials for different tasks. Here, we used poly(D,L-lactic-co-glycolic acid) (PLGA), a well-known biodegradable and biocompatible polymer. The diameter of the TMMC depends on the diameter of the blood vessel that will be used for embolization where the drug will be released.
Q What is the relationship between catheter proximity to the bifurcation and flow velocity?
A Three main parameters must be taken into account, namely, the blood flow, the distance to the next bifurcation, and the response of the TMMC to the magnetic gradients (a higher percentage of nanoparticles leads to faster steering). For a given TMMC loaded with a given percentage of magnetic nanoparticles, a sufficiently long distance must be provided prior to the next bifurcation to allow enough time to steer all the TMMC toward the right branch at the next bifurcation. A higher blood-flow rate would reduce the time available for steering the TMMC. Then more nanoparticles in each TMMC can be used, which would reduce the percentage of therapeutics in each TMMC, and/or the catheter used to release the TMMC can be placed so as to provide more distance and hence more time prior to the next bifurcation.
Q What is the desired timeline for polymer degradation?
A The drug-release profile (here, doxorubicin) was based on drug-eluting beads (DEB) design with degradation over several days. Other types of release are also possible but need further investigation, such as using different synthesis processes and, in some other cases, using external triggering methods.
Q Does the polymer limit the targeting, and should care be taken in which type of polymer is used?
A We observed no unexpected interactions between the polymer, the nanoparticles, and the cytotoxic agent. The polymer controlling release through biodegradation did not limit the targeting. Indeed, the choice of the polymer is a critical issue in the synthesis of TMMC. In this study, we used biodegradable PLGA.
Q What happens to the magnetic nanoparticles once they have been released from the polymeric carrier?
A That is a good question. Presently, two main types of nanoparticles are being considered by our group. One is iron–cobalt nanoparticles and the other is iron oxide nanoparticles. In this study, iron–cobalt nanoparticles have been used. Iron–cobalt nanoparticles have a much (almost three times) higher magnetization saturation level than do iron oxide nanoparticles of the same dimensions. This higher saturation level translates, in an engineering point of view, to a higher performance-induced propelling force and hence to faster and more efficient steering of TMMC to the targeted branch at vessel bifurcation. To maintain saturation magnetization level of the iron–cobalt nanoparticles, a thin layer of graphite only a few nanometers in thickness was synthesized to prevent oxidation. Because of this new type of nanoparticle, we do not know yet what happens to these nanoparticles once in the body, and further investigations on this matter would be necessary. On the other hand, iron oxide nanoparticles can also be used (but leading to less steering performance), and since these nanoparticles have been intensively used as contrast agents for MRI, they are probably, for now, a safer choice until we know more about iron–cobalt nanoparticles.
Q How do you explain the higher steering efficiency for left steering compared with right steering?
A To keep it short and without going into too many technical details, I would say that since the TMMC characteristics were the same, the gradients applied were the same, and the catheter release sites were the same, then this can be explained by differences in vascular-related physiological conditions between the two lobes affecting the steering of the TMMC.
Q Do you have any plans to further optimize the design to achieve greater effect (e.g., steering efficiency)?
A Yes, we have plans to increase the steering efficiency, and we are presently investigating several of these to enhance such targeting. To answer the second question, we perform the tests on rabbits using the same gradient magnitude that would be technologically and physiologically possible on humans. I must say that such steering is somewhat more difficult in rabbit models considering the blood flows, shorter distance before the next bifurcation, and use of smaller TMMC and, hence, smaller amount of magnetic material.
Q Do you expect the design of the TMMCs to be altered in any form for clinical application in humans?
A For humans, we typically have a longer distance prior to the next bifurcation and larger arteries than for rabbits, which suggest the use sensibly larger TMMC with more magnetic nanoparticles. Then, combining longer distances and more steering-responsive TMMC to magnetic gradients suggests indeed better targeting for humans compared with rabbits. But this hypothesis still needs to be validated at some point in the future.
Q What major hurdles remain to be overcome in this technology before clinical application?
A Indeed, the two major challenges are to increase the number of bifurcations that such TMMC can navigate effectively and, second, to reach the tumor in some other types of interventions instead of chemoembolization used here for the liver. We have shown so far that we can not only encapsulate a drug in MRI-navigable (referred to here as MRN) microcarriers (the TMMC) as small as 50 micrometers in diameter and release it at a targeted site but also that we can generate sufficient magnetic gradient for future operation in humans for effective targeting. But presently, the technology is relatively slow at changing the direction of the TMMC, and considering that the distance between successive bifurcations decreases as we go deeper in the vascular network, accessing some targeted sites located deeper in the vascular network becomes very challenging. As such, we are presently investigating techniques and new complementary approaches to allow navigation of TMMC across several bifurcations. For targeting inside the tumors, our previous experimental data and our theoretical models show that this approach would be limited to TMMC larger than approximately 50 micrometers, which is too large to target inside the tumors. Ideally, microcarriers of 2 micrometers in diameter with sufficient thrust propelling force, which is impossible to induce for a TMMC at such a scale, would be necessary. As such, we are using a special bacterium (2 micrometer diameter), propelled by flagellated motors and fully controllable by computer, that proved to be very effective in the microvasculature and the angiogenesis network that provides an entrance to the tumoral site. We are presently developing within a pharmaceutical consortium a new therapeutic agent based on these bacteria to treat colorectal cancer. Unfortunately, these bacteria are less effective in larger blood vessels due to larger blood flows and too long a distance to travel. We are presently investigating the encapsulation of these bacteria in special microcarriers (with characteristics similar to the TMMC) acting as “submarines” being steered (navigated) using the same MRI navigation technique to transport and to release these bacteria carrying the therapeutic load closer to the microvasculature to deliver their loads deep inside tumors.
Reference
Pouponneau, P, Leroux, JC, Soulez, G, Gaboury, L, Martel, S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation, Biomaterials 32: 3481–3486 (2011).
Links
The publication: http://www.sciencedirect.com/science/article/pii/S0142961211000780
Professor Martel’s website: http://wiki.polymtl.ca/nano/index.php/Biography