By Hardik Shah
About Physiolution
Physiolution GmbH was founded by Prof. Dr. Werner Weitschies and Dr. Grzegorz Garbacz as a spin-off of the University of Greifswald in 2009. Physiolution is a highly innovative company focused on investigations of the dissolution behaviour of solid oral dosage forms under biorelevant test conditions.
The cutting-edge test methods Physiolution utilizes are based on long experience in the fields of gastrointestinal (GI) tract physiology and biopharmaceutical evaluation of dosage form performance. The biorelevant test devices realistically simulate the mechanistic and physicochemical conditions that act on dosage forms during their GI passage and enable the evaluation of their biopharmaceutical safety and reliability. In addition, Physiolution’s analytical services include investigations of mechanical stability of solid oral dosage forms and development and validation of analytical methods and procedures for the determination of the dissolution characteristics of dosage forms and APIs under biorelevant test conditions. The applicability of the test devices and test procedures was successfully validated on numerous examples of clinical relevance as well as in the routine analyses of solid oral dosage forms. For more details, please visit www.Physiolution.eu or contact Dr. Grzegorz Garbacz, ggarbacz@physiolution.eu.
Q One specialty area of Physiolution is investigation of dissolution behaviour of solid oral dosage forms under biorelevant conditions. In your opinion, what are the drawbacks of the current testing methods?
A In the last few decades dissolution testing has become a powerful tool for quality control and is also frequently applied for determination of the (bio)pharmaceutical equivalence of different products. The compendial dissolution tests have a relatively simple construction and provide well-definable conditions by implying continuous exposure of the dosage form to a sufficient amount of dissolution medium and mechanical agitation. The most relevant dissolution test devices for the investigation of solid oral dosage forms are Apparatuses 1–4 of the USP and European Pharmacopoeia. These devices represent highly standardized tools for quality control, and with appropriate experimental settings the simulation of the physicochemical conditions in the GI tract is also possible.
However, these well definable and continuous conditions during the dissolution test do not reflect the physiological circumstances along the GI tract. The design of the official dissolution test apparatus does not provide the possibility of simulating GI mechanical stress conditions in a realistic way and does not reflect the volumes, discontinuous distribution, and flow patterns of the GI fluids [1–4]. The key factors of physical conditions of the GI passage that may affect the drug delivery behaviour of solid oral dosage forms and that are not considered by the compendial dissolution tests are as follows:
- the discontinuity of the movement of dosage forms along the GI tract with peak velocities of up to 50 cm/s during gastric emptying and ileocaecal passage [5]
- the GI motility in the form of sequences of mechanical contractions with an intensity of up to 300 mbar [6–9]
- the intermittent contact of the dosage form with GI fluids [10]
- the physiological volume changes of liquids and their flow patterns such as gastric emptying rates specific for different prandial conditions and patient groups [11, 12]
- the intragastric temperature gradients characteristic under fasting conditions [1, 13]
Q What do you mean “biorelevant” condition? How do you simulate the mechanistic condition of the GI?
A It is known that the release behaviour of solid oral dosage forms in the GI tract can also be affected by mechanical stress. It has been recognized that the GI transit of dosage forms is characterized by highly variable conditions with long rest phases and short but intensive events of transport. During GI transport events, dosage forms are moved with high velocities of up to 30–50 cm/s for short periods of time. Such intensive movements are mainly triggered by gastric emptying and transition through the ileocaecal junction and colonic mass movement. During GI transit solid dosage forms are also exposed to mechanical pressure caused by GI motility events. Maximum pressures are registered in the antropyloric region of the stomach and in the ileocaecal region and reach up to 300 mbar in the case of monoliths such as modified release (MR) tablets [4, 6]. It has already been demonstrated that the release behaviour of targeted MR formulations can be affected by mechanical stress in the GI tract [2–4, 14–16].
Considering the complex physiology of the GI tract, standard dissolution methods are not necessarily capable of simulating the GI transit conditions of solid oral dosage forms realistically. By using the biorelevant dissolution stress test device developed by Physiolution’s founders, the impact of mechanical stress on drug release of MR products can be investigated [2–4]. The device simulates essential physiological stress parameters including discontinuous dosage form movement and GI motility forces using physiology-based test algorithms. By this, the stress test device can demonstrate the mechanical conditions of GI transit in a biorelevant way.
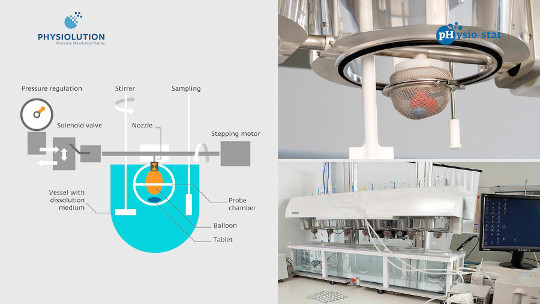
The dissolution stress test device enables a biorelevant simulation of the physiological mechanical stress that may occur during GI passage of a solid dosage form. During the tests the dosage forms are exposed to sequences of agitation including movement and pressure fluctuations as well as phases of rest. The intensity, timing, and duration of the subsequent test phases are in accordance with the circumstances in vivo.
Briefly, the dissolution stress test device consists of a central apparatus axle with seven spheres (probe chambers) made of a stainless steel wire netting in which the dosage forms are hosted throughout the test. Each sphere is divided into two parts. The bottom part is screwed into the central pipe-like axle by a bush and a nozzle. In the working mode, the central pipe is placed on the deck plate of the device about 3 mm above the top edges of a row of seven standard dissolution vessels in their symmetry plane. Consequently, each sphere operates in a separate vessel. On one end the central axis is connected to a pressure regulation device and on the other end to a stepping motor. Pressure waves are generated by periodic inflation and deflation of the balloons located inside the probe chambers. The inflation and deflation of the balloons are controlled by switching of valves, whereas the occurring pressure can be regulated by a pressure-reducing device. The central axis is driven by the stepping motor, which is computer controlled and enables a programmable movement and positioning of the probe chambers. All test parameters are controlled by custom-made software [4].
 |
 |
Q How about the physiochemical conditions?
A The realistic simulation of the physicochemical conditions of the GI tract is a prerequisite for good estimation of the drug delivery behaviour of oral dosage forms. As mentioned before, the standard dissolution tests with appropriate experimental settings also enable simulating at least to some extent the physicochemical conditions in the GI tract. It has already been recognized that a set of dissolution media representing changing pH conditions in the GI sections that are relevant for drug release is often sufficient to predict the in vivo performance of oral dosage forms. Results from such experiments are typically even more predictive if such buffers are adapted to represent not only the pH but also the osmolality and ionic strength of the GI contents. However, simple aqueous buffers often do not represent other key aspects of the composition of the GI contents, including the surface tension, the viscosity, and the presence of food or digestive products, which can be relevant to drug release from the tested dosage form [2]. Therefore, various biorelevant media simulating conditions in the fasted or fed stomach, small intestine, and colon have been proposed and successfully used in the last decade [17–23]. The most current scientific findings in human GI physiology have resulted in a recent update of these media to represent both the pre- and postprandial states more closely.
Interestingly, to date the composition and volume of the media recommended for the biorelevant simulation of the human GI tract does not undergo continuous temporal changes and so does not reflect physiological media processing such as digestion, acidification, or temperature changes. In the context of recent research, the hydrogen carbonates as most physiological buffer species and the overall buffer capacity of the dissolution media play an essential role for the intraluminal dissolution of ionizable compounds such as drugs, polymers, and formulations thereof [24–28].
Q What are the challenges of using hydrogen carbonate buffers? How do you address the instability issue of the gas in the dissolution media? How do you control the pH, ionic strength, and so on?
A The hydrogen carbonate buffer is considered the most biorelevant buffer system for the simulation of intestinal conditions and covers the physiological pH range of the luminal fluids from pH 5.0 to about pH 8.4. However, its use for the in vitro evaluation of the dissolution performance of solid oral dosage forms is limited. This is mostly due to its thermodynamic instability resulting in an uncontrolled carbon dioxide loss followed by pH increase [24–26, 29–31].
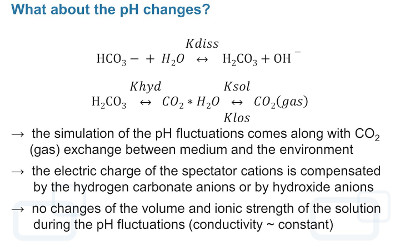
 The pH value of a hydrogen carbonate buffer is the result of a complex and highly dynamic interplay of the concentration of hydrogen carbonate ions, carbonic acid, dissolved and solvated carbon dioxide, and its partial pressure above the solution. The complex equilibrium between the different ions results in a thermodynamic instability of hydrogen carbonate solutions. To maintain the pH value, it is necessary to prevent carbon dioxide losses (hardly manageable under routine test conditions) or to replace the escaping carbon dioxide by feeding equivalent gas volumes into the solution to reacidify the buffer system [27, 28].
The pH value of a hydrogen carbonate buffer is the result of a complex and highly dynamic interplay of the concentration of hydrogen carbonate ions, carbonic acid, dissolved and solvated carbon dioxide, and its partial pressure above the solution. The complex equilibrium between the different ions results in a thermodynamic instability of hydrogen carbonate solutions. To maintain the pH value, it is necessary to prevent carbon dioxide losses (hardly manageable under routine test conditions) or to replace the escaping carbon dioxide by feeding equivalent gas volumes into the solution to reacidify the buffer system [27, 28].
 At a first sight, the thermodynamic instability of hydrogen carbonate buffers can be considered a substantial disadvantage. However, it offers the unique opportunity to use a physiological buffer system that provides the possibility of simulating the dynamic intraluminal pH changes in the human small intestine and colon. To do so, both processes—the acidification as well as the alkalisation of the buffer system—require continuous and dynamic adjustment. It is well known that the acidification of the commonly used hydrogen carbonate buffers is a rather fast process, whereas the carbon dioxide loss occurs relatively slowly and spontaneously.
At a first sight, the thermodynamic instability of hydrogen carbonate buffers can be considered a substantial disadvantage. However, it offers the unique opportunity to use a physiological buffer system that provides the possibility of simulating the dynamic intraluminal pH changes in the human small intestine and colon. To do so, both processes—the acidification as well as the alkalisation of the buffer system—require continuous and dynamic adjustment. It is well known that the acidification of the commonly used hydrogen carbonate buffers is a rather fast process, whereas the carbon dioxide loss occurs relatively slowly and spontaneously.
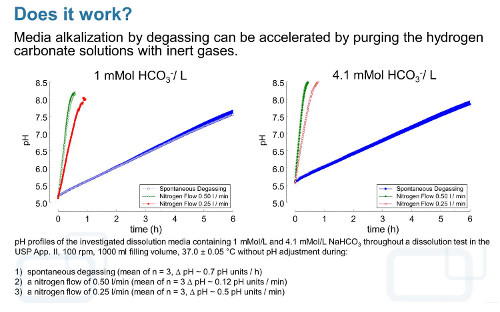
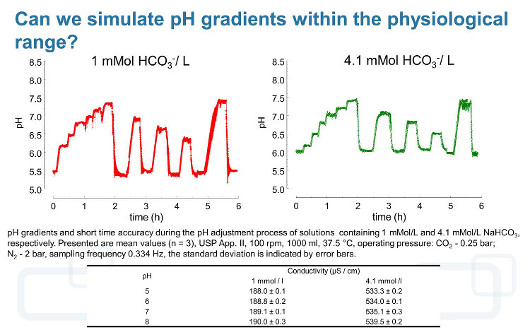
However, under dissolution test conditions the degassing process can be accelerated and controlled by purging the solution with an inert gas such as N2. If this inert gas is introduced into the dissolution medium, it enlarges the surface available for gas exchange and increases the carbon dioxideevacuation rate, which results in a rapid increase of the pH value. During pH adjustment of hydrogen carbonate buffer media by using carbon dioxide, neutral gases, or mixtures thereof, the resulting pH changes come along with carbon dioxide (gas) exchange between the medium and environment. Because the charge of cations from strong bases such as K+ or Na+, which are commonly used as hydrogen carbonate salts, is compensated by HCO3– during acidification or OH– resulting from water hydrolysis during alkalisation, the total ion concentration and so the conductivity and ionic strength remain almost constant within an experiment in the case of solutions containing only monovalent ions or ions of weak electrolytes. Therefore, with a well-designed and well-controlled hydrogen carbonate buffer system, it should be possible to adequately simulate intraluminal pH conditions and ionic composition in an in vitro experiment [28].
In order to use hydrogen carbonate buffers with pH gradients in the physiological range and with the dynamics observed in vivo without changing the ionic strength of the solution, we developed a device (pHysio-grad®) that provides both acidification of the dissolution medium by microcomputer-controlled carbon dioxide influx and alkalisation by degassing [28].
Q What types of drugs are uniquely suited to use the hydrogen carbonate buffers (where a traditional stable buffer system fails to be relevant)?
A The application of the hydrogen carbonate buffer system is essential for a realistic simulation of intestinal conditions, particularly if it is the aim to predict drug release from solid oral dosage forms containing ionisable drugs and/or excipients and estimate the realistic dissolution rate of the raw materials. The dissolution of such formulations can be significantly altered by the ionic composition, total ion concentration, and buffer capacity of the dissolution media. It has been demonstrated that, for dissolution testing of dosage forms containing ionisable compounds, hydrogen carbonate buffers are often more discriminative than compendial phosphate buffers. For this reason, in various cases they enable a better estimation of in vivo drug release based on in vitro results [24–26, 28, 30, 32].
Also, in the case of enteric coated formulations as well as formulations comprising ionic polymers, the choice of an adequate buffer system is crucial for the predictive value of the dissolution experiment. It has been demonstrated for various test formulations that by using hydrogen carbonate buffers a more realistic estimation of the in vivo drug release characteristics can be achieved.
It is likely that the use of other buffer species such as phosphates, citrates, maleates, tartrates, and so on as solutions of buffer capacity or ionic strength corresponding to commonly used hydrogen carbonate media in some cases may lead to similar dissolution results. However, from our point of view it cannot be generally recommended. It should be kept in mind that the relationship between the dissolution profiles/dissolution rate and the buffer species as well as the ionic strength of the solution is a result of complex interplay of formulation characteristics as well as properties of the API. Without a deep understanding of the formulation principles, composition, and technology (as, for example, during characterization of commercial products) it is hard to justify whether the dissolution profiles obtained using diluted compendial buffer species will correspond to the performance of the particular formulation in the more physiological hydrogen carbonate media.
Q In different GI regions, pH conditions vary, which is particularly important for a controlled (modified) release system. How do you address this type of dynamic condition with the hydrogen carbonate buffer system?
A The main problem with the simulation of the in vivo dynamics of the pH changes along the intestines is the substantial lack of the availability of individual intraluminal pH profiles of sufficiently high resolution. To date, several techniques are available for the evaluation of GI pH conditions; however, only telemetric capsules such as IntelliCap® (Medimetrics, Eindhoven, The Netherlands) enable real-time pH and temperature monitoring along the GI tract. The profiles obtained in such studies after processing are used as an input function for the simulation of the intraluminal conditions. We hope to contribute on this interesting topic in the very near future.
As mentioned above, the simulation of the intraluminal pH changes can be successfully performed using hydrogen carbonate buffers. For this purpose we are using the thermodynamic instability of the buffer system, which offers the unique opportunity to simulate the dynamic pH fluctuations without changing the volume and ionic strength of the solution. This requires that both processes (the acidification by introducing the carbon dioxide into the solution as well as the alkalisation of the buffer system by purging an inert gas) require continuous and dynamic adjustment. This task can be performed using automated controllers such as our pHysio-grad® device with high accuracy and resolution [28].
References
1. Garbacz, G, Cadé, D, Benameur, H, Weitschies, W. Bio-relevant dissolution testing of hard capsules prepared from different shell materials using the dynamic open flow through test apparatus, Eur. J. Pharm. Sci. 57: 264-272 (2013).
2. Garbacz, G, Klein, S. Dissolution testing of oral modified-release dosage forms, J. Pharm. Pharmacol. 7: 944-968 (2012).
3. Garbacz, G, Klein, S, Weitschies, W. A biorelevant dissolution stress test device—Background and experiences, Expert Opin. Drug Deliv. 11: 1251-1261 (2010).
4. Garbacz, G, Wedemeyer, RS, Nagel, S, Giessmann, T, Mönnikes, H, Wilson, CG, Siegmund, W, Weitschies, W. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses, Eur. J. Pharm. Biopharm. 2: 421-428 (2008).
5. Weitschies, W, Kosch, O, Mönnikes, H, Trahms, L. Magnetic marker monitoring: An application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms, Adv. Drug Deliv. Rev. 8: 1210-1222 (2005).
6. Cassilly, D, Kantor, S, Knight, LC, Maurer, AH, Fisher, RS, Semler, J, Parkman, HP. Gastric emptying of a non-digestible solid: Assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy, Neurogastroenterol. Motil. 4: 311-319 (2008).
7. Kamba, M, Seta, Y, Kusai, A, Ikeda, M, Nishimura, K. A unique dosage form to evaluate the mechanical destructive force in the gastrointestinal tract, Int. J. Pharm. 1-2: 61-70 (2000).
8. Kamba, M, Seta, Y, Kusai, A, Nishimura, K. Comparison of the mechanical destructive force in the small intestine of dog and human, Int. J. Pharm. 1-2: 139-149 (2002).
9. Dinning, PG, Bampton, PA, Kennedy, ML, Kajimoto, T, Lubowski, DZ, de Carle, DJ, Cook, IJ. Basal pressure patterns and reflexive motor responses in the human ileocolonic junction, Am. J. Physiol. 276: G331-G340 (1999).
10. Barale, V, Schiller, C, Tacchi, R, Marechal, C. Trends and interactions of physical and bio-geo-chemical features in the Adriatic Sea as derived from satellite observations, Sci. Total Environ. 1-3: 68-81 (2005).
11. Koziolek, M, Garbacz, G, Neumann, M, Weitschies, W. Simulating the postprandial stomach: Physiological considerations for dissolution and release testing, Mol. Pharm. 5: 1610-1622 (2013).
12. Koziolek, M, Grimm, M, Garbacz, G, Kühn, JP, Weitschies, W. Intragastric volume changes after intake of a high-caloric, high-fat standard breakfast in healthy human subjects investigated by MRI, Mol. Pharm. 5: 1632-1639 (2014).
13. Sun, WM, Houghton, LA, Read, NW, Grundy, DG, Johnson, AG. Effect of meal temperature on gastric emptying of liquids in man, Gut 3: 302-305 (1988).
14. Garbacz, G, Golke, B, Wedemeyer, RS, Axell, M, Söderlind, E, Abrahamsson, B, Weitschies, W. Comparison of dissolution profiles obtained from nifedipine extended release once a day products using different dissolution test apparatuses, Eur. J. Pharm. Sci. 2: 147-155 (2009).
15. Garbacz, G, Kandzi, A, Koziolek, M, Mazgalski, J, Weitschies, W. Release characteristics of quetiapine fumarate extended release tablets under biorelevant stress test conditions, AAPS PharmSciTech 15(1): 230-236 (2013).
16. Garbacz, G, Weitschies, W. Investigation of dissolution behavior of diclofenac sodium extended release formulations under standard and biorelevant test conditions, Drug Dev. Ind. Pharm. 5: 518-530 (2010).
17. Kalantzi, L, Goumas, K, Kalioras, V, Abrahamsson, B, Dressman, JB, Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies, Pharm. Res. 1: 165-176 (2006).
18. Diakidou, A, Vertzoni, M, Abrahamsson, B, Dressman, J, Reppas, C. Simulation of gastric lipolysis and prediction of felodipine release from a matrix tablet in the fed stomach, Eur. J. Pharm. Sci. 2: 133-140 (2009).
19. Diakidou, A, Vertzoni, M, Dressman, J, Reppas, C. Estimation of intragastric drug solubility in the fed state: Comparison of various media with data in aspirates, Biopharm. Drug Dispos. 6: 318-325 (2009).
20. Diakidou, A, Vertzoni, M, Goumas, K, Söderlind, E, Abrahamsson, B, Dressman, J, Reppas, C. Characterization of the contents of ascending colon to which drugs are exposed after oral administration to healthy adults, Pharm. Res. 9: 2141-2151 (2009).
21. Vertzoni, M, Fotaki, N, Kostewicz, E, Stippler, E, Leuner, C, Nicolaides, E, Dressman, J, Reppas, C. Dissolution media simulating the intralumenal composition of the small intestine: Physiological issues and practical aspects, J. Pharm. Pharmacol. 4: 453-462 (2004).
22. Jantratid, E, Janssen, N, Reppas, C, Dressman, JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: An update, Pharm. Res. 7: 1663-1676 (2008).
23. Abrahamsson, B, Roos, K, Sjögren, J. Investigation of prandial effects on hydrophilic matrix tablets, Drug Dev. Ind. Pharm. 6: 765-771 (1999).
24. Fadda, HM, Merchant, HA, Arafat, BT, Basit, AW. Physiological bicarbonate buffers: Stabilisation and use as dissolution media for modified release systems, Int. J. Pharm. 1-2: 56-60 (2009).
25. Ibekwe, VC, Fadda, HM, McConnell, EL, Khela, MK, Evans, DF, Basit, AW. Interplay between intestinal pH, transit time, and feed status on the in vivo performance of pH responsive ileo-colonic release systems, Pharm. Res. 8: 1828-1835 (2008).
26. Liu, F, Merchant, HA, Kulkarni, RP, Alkademi, M, Basit, AW. Evolution of a physiological pH 6.8 bicarbonate buffer system: Application to the dissolution testing of enteric coated products, Eur. J. Pharm. Biopharm. 1: 151-157 (2011).
27. Garbacz, G, Kołodziej, B, Koziolek, M, Weitschies, W, Klein, S. An automated system for monitoring and regulating the pH of bicarbonate buffers, AAPS PharmSciTech 2: 517-522 (2013).
28. Garbacz, G, Kołodziej, B, Koziolek, M, Weitschies, W, Klein, S. A dynamic system for the simulation of fasting luminal pH-gradients using hydrogen carbonate buffers for dissolution testing of ionisable compounds, Eur. J. Pharm. Sci. 51: 224-231 (2014).
29. Sheng, JJ, McNamara, DP, Amidon, GL. Toward an in vivo dissolution methodology: A comparison of phosphate and bicarbonate buffers, Mol. Pharm. 1: 29-39 (2009).
30. Ibekwe, VC, Liu, F, Fadda, HM, Khela, MK, Evans, DF, Parsons, GE, Basit, AW. An investigation into the in vivo performance variability of pH responsive polymers for ileo-colonic drug delivery using gamma scintigraphy in humans, J. Pharm. Sci. 12: 2760-2766 (2006).
31. McConnell, EL, Fadda, HM, Basit, AW. Gut instincts: Explorations in intestinal physiology and drug delivery, Int. J. Pharm. 2: 213-226 (2008).
32. Varum, FJ, Hatton, GB, Freire, AC, Basit, AW. A novel coating concept for ileo-colonic drug targeting: Proof of concept in humans using scintigraphy, Eur. J. Pharm. Biopharm. 3: 573-577 (2013).
Copyright 2014


