By Sebastien Boridy (McGill University, Montreal, Canada)
By Sebastien Boridy (McGill University, Montreal, Canada)
For close to 40 years, CRS has been devoted to spreading information and knowledge about the science and technology of controlled release. In light of this history, Inside Track has decided to highlight the work of longtime CRS members to commemorate their dedication to the society and its mission. It is therefore only fitting that we start this series with a member who has served CRS for over two decades: Dr. Hamid Ghandehari.
Dr. Ghandehari is currently a professor in the departments of Pharmaceutics and Pharmaceutical Chemistry and Bioengineering at the University of Utah, U.S.A., and he is the director of the Utah Center for Nanomedicine, part of the Nano Institute of Utah, which he cofounded. Dr. Ghandehari’s groupdevelops novel methods for controlled delivery of bioactive agents, mainly focusing on genetically engineered polymers for gene delivery, water-soluble polymers for targeted delivery, poly(amido amine) (PAMAM) dendrimers for oral delivery, and inorganic nanoconstructs for controlled chemical delivery.
Dr. Ghandehari has served in leadership roles in CRS and the broader drug delivery community. He was the program cochair of the bioactive materials track during the 2012 annual meeting, established the CRS Nanomedicine Focus Group (and served as its chair until 2012), and has been nominated for the CRS Board of Scientific Advisors in 2014. Dr. Ghandehari was an active member of the CRS Young Scientist Award Committee from 1999 to 2005 and has chaired several CRS sessions, roundtables, workshops, and Pearls of Wisdom sessions throughout the years.
As editor-in-chief of Advanced Drug Delivery Reviews, Dr. Ghandehari has a unique perspective on developments in the field of polymeric drug delivery. We recently got the opportunity to interview him and discuss the current state of affairs, as well as where we’re headed.
Q The field of polymer therapeutics, a term coined by Dr. Ruth Duncan, has come a long way in the past 20 years. Which key breakthroughs do you believe set the stage for our present successes?
A A lot of pioneering work was done in the late 1970s and throughout the 1990s to establish the biocompatibility and pharmacokinetics of the carrier macromolecules, spacer chemistry for drug release, and targeting strategies. Key breakthroughs, I think, have been the discovery of the enhanced permeability and retention effect (EPR), the evolution of our understanding of the subcellular fate of macromolecular therapeutics, and the first clinical trial for N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin conjugates in the mid-1990s, among others. Although these systems did not advance beyond phase II, HPMA copolymer-doxorubicin conjugates were an example of an early prototype, and lessons learned from these conjugates have been applied for design of new delivery systems. These include the importance of the size of macromolecules, design of spacer chemistries that allow rapid and more complete release at the target site, development of synthetic strategies that enable the production of polymers with low polydispersity, and finally, development of systems that are eliminated from the body by biodegradation and elimination. Despite a tremendous amount of basic and clinical research over the past four decades, I think there are still substantial challenges for the successful development of polymer therapeutics. These include the limited penetration of macromolecular carriers in solid tumors, heterogeneity in target tissue among the patient population, and the myriad of degradation products that complicate the analysis of these systems for successful review and approval by regulatory bodies.
Q Localized tumor hyperthermia using gold nanoparticles has been shown to promote delivery of targeted copolymers to solid tumors in some of your most recent work. What advantages does this method afford over delivery of polymers to naïve (untreated) tumor tissue?
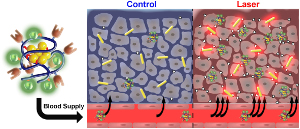
A Plasmonic photothermal therapy using gold nanoparticles provides the opportunity for mild hyperthermia in solid tumors in a controlled fashion. This results in increased blood flow and expression of a subset of receptors, which in turn enhance the delivery of polymer-drug conjugates. Our research group has shown that using this method leads to increased delivery of water-soluble polymer-drug conjugates to prostate tumors in a synergistic manner.
|
|
|
Researchers in the Ghandehari group have shown that mild hyperthermia resulted from gold nanorod mediated plasmonic photothermal therapy enhances the delivery of polymer therapeutics to solid tumors. Reprinted from Nano Today, Volume 7, Issue 3, A. J. Gormley, N. Larson, S. Sadekar, R. Robinson, A. Ray, and H. Ghandehari, Guided Delivery of Polymer Therapeutics Using Plasmonic Photothermal Therapy, Pages 158-167, Copyright 2012, with permission from Elsevier. http://www.sciencedirect.com/science/journal/17480132 |
Q Some of the work at the Utah Center for Nanomedicine focuses on design and development of recombinant polymers for gene delivery. What properties make such systems promising for controlled delivery of bioactive agents?
A The majority of polymers used in controlled release to date are synthesized by traditional chemical techniques. Such techniques result in the production of polymers with limited control over the sequence of monomers and polydispersity. Recombinant techniques have enabled the production of protein-based polymers with precise sequences and lengths. This approach provides a precise correlation between structure and function as it pertains to controlled release. Collaborative work in our lab focuses on the design and development of recombinant silk-elastin like copolymers for matrix-mediated delivery of adenoviral gene delivery systems. These polymers are liquid at room temperature, can be mixed with bioactive agents, and form hydrogels at body temperature. By precisely controlling the lengths and sequences of silk and elastin blocks, we have shown that the delivery and transfection efficiency of genetic material can be controlled. Our most recent work involves the introduction of matrix-metalloproteinase responsive sequences at precise locations in the polymer backbone. I think this synthetic strategy holds tremendous potential for the development of bioresponsive polymers. At the moment, the small scientific community working in this area is barely scratching the surface.
Q Your lab has worked on evaluation of PAMAM dendrimers for oral drug delivery. In your opinion, what are the strengths and limitations of these constructs for oral delivery?
A Over the years, we have studied the influence of size, surface charge, and incubation time of PAMAM dendrimers on transepithelial transport and toxicity. This work has established some trends. We now know that, in general, cationic dendrimers are more toxic than anionic and hydroxyl-terminated dendrimers; an increase in concentration and incubation time can increase potential for toxicity; the dendrimers are transported by a combination of paracellular and transcellular routes; and that compared with poly(ethylene glycol) or dextrans of similar molecular weights, PAMAM dendrimers are transported to a higher extent across epithelial barriers. For example, more recent studies have shown that anionic PAMAM dendrimer generation 6.5 has single-digit oral bioavailability. These results suggest that there is a window of opportunity to design PAMAM dendrimers as scaffolds for oral delivery of polymer therapeutics. However, substantial challenges remain. Among these challenges is the fact that large doses of the carriers are required to exert their effect. Spacer chemistries need to be developed to provide stability in the GI tract and bloodstream, while enabling release at the target site. In addition, more detailed mechanistic studies are needed to pinpoint the mechanisms of transport. Finally, experiments need to be designed to correlate in vitro and in vivo studies and ultimately human applications.
Q Some of the more recent work in your research group focuses on nanotoxicology. What prompted this work, and how do you see the evolution of this burgeoning field?
A Advances in nanotechnology have provided the opportunity to make nanomaterials with defined shapes and surface properties. Less is known, however, on how these physicochemical properties influence biological fate. For example, there is a renewed interest in the use of inorganic materials such as silica and gold nanoparticles in drug delivery applications. Recent work in our lab focuses on understanding the influence of the shape, surface charge, and porosity of these particles on mechanisms of cellular uptake, biodistribution, and toxicity. Work to date points to the crucial influence of surface properties and porosity. Although geometry does seem to have some effect in the size ranges we have studied (below 500 nm), this effect is limited compared with surface characteristics. Substantial challenges remain to understand the influence of these physicochemical properties on protein adsorption, stimulation of immune response, chronic toxicity, development of biodegradable inorganic nanoparticles, and understanding their biological fate, among other factors.
Q There’s been a longstanding debate regarding the cost effectiveness of active targeting, with valid arguments for and against. How do you view this debate and current investments in receptor-targeted drug delivery systems?
A In my view, this subject needs to be studied on a case-by-case basis, and blanket statements on whether active targeting is useful or not are not helpful. For example, the architecture of the carrier, location of the target cells, type of targeting moieties, and spacers linking them to the carrier are among important factors that influence success in active targeting. While I am not a specialist in forecasting the cost effectiveness of such an approach, I think active targeting strategies that target specific niches to result in substantial improvement of efficacy and reduction of toxicity, hence improving patients’ lives, are worth pursuing.
Q As editor-in-chief of Advanced Drug Delivery Reviews, you have helped guide the field to where it is today. What advances do you foresee over the next 10 years for drug delivery research?
 |
|
The Ghandehari lab group |
A Advances in drug delivery stem from development of new materials, a better understanding of biological mechanisms, and successful clinical translation. I think we will see newer and better defined materials in terms of structure, shape, polydispersity, bioresponsiveness, and the like. Despite tremendous advances in our understanding of biodistribution and subcellular fate of common materials used in delivery of bioactive agents, this field is still wide open, and in collaboration with biologists, we need to better understand how to target subcellular pathways with our new or existing delivery approaches. Finally, I think continued involvement of clinicians in our field is essential to enable us to address real unmet needs with our toolbox of controlled release materials. I appreciate the support that many colleagues have provided to Advanced Drug Delivery Reviews over the years by submitting outstanding reviews and theme issues as well as participating in the review process. And, of course, I always welcome continued interest and new ideas for theme issues.
Q If you had $1 million added to your research budget tomorrow, what question would you invest most of your time investigating?
A I am afraid to say that with the rising cost of biomedical research, $1 million would not go too far, but of course given the tragic dwindling of federal funding, I would welcome it! Here are some of the questions that I think our research group would like to answer: Can we use alternative physical means to provide hyperthermia to solid tumors for enhancing the delivery of polymer therapeutics? And how would physicochemical properties of silica nanoconstructs modulate inflammatory responses and chronic toxicity?
Q What advice would you give to young scientists starting out on their careers?
A Enthusiasm is the fuel for advancement of science. Coupled with hard work, persistence, and innovation, these are the key elements that a successful scientist needs. If we look at the history of controlled release science, for example, some of the most exciting developments have occurred at the interface of materials science and biology. I think looking outward to observe new findings in the basic sciences that seemingly don’t relate to your field of research, and applying these concepts to come up with new and improved delivery strategies, is the key to innovation. For young scientists embarking on an academic career, don’t be afraid of the limited funding environment. There is always the opportunity for cutting-edge research, and the community needs you more than ever.
Q Are there any relevant articles or papers that you would recommend?
A Related to polymer therapeutics, in a series of recent review articles I think Ruth Duncan and coworkers have done a great job in articulating the state of the art and the challenges ahead in this field. For more information on some of the research that was described above, the following articles provide a small taste:
1) Gormley, AJ, Larson, N, Sadekar, S, Robinson, R, Ray, A, Ghandehari, H. Guided Delivery of Polymer Therapeutics Using Plasmonic Photothermal Therapy, Nano Today 7(3): 158-167 (2012).
2) Frandsen, JL, Ghandehari, H. Recombinant Protein-Based Polymers for Advanced Drug Delivery, Chem. Soc. Rev. 41(7): 2696-2706 (2012).
3) Greish, K, Thiagarajan, G, Herd, H, Price, R, Bauer, H, Hubbard, D, Burckle, A, Sadekar, S, Yu, T, Anwar, A, Ray, A, Ghandehari, H. Size and Surface Charge Significantly Influence the Toxicity of Silica and Dendritic Nanoparticles, Nanotoxicology 6(7): 713-723 (2012).
4) Sadekar, S, Ghandehari, H. Transepithelial Transport and Toxicity of PAMAM Dendrimers: Implications for Oral Drug Delivery, Adv. Drug Deliv. Rev. 64(6): 571-588 (2012).
Copyright 2014