By Vishwas Rai, Ph.D., Chrono Therapeutics, Waltham, MA, U.S.A.
“I worked at Rutgers University for the last 28 years, but I feel as if I’ve had four completely different jobs during those 28 years, ” said Joachim Kohn when I asked him about his academic journey. He continued: “I had a wonderful career in academia, full of challenges but always very exciting. But now I see a lot of change, and I expect that the lives of all the young scientists who are only now starting out will be very different from the experiences I had over the last 30 years of my academic journey.”
Today, Joachim Kohn is widely recognized as a leader in biomaterials science. Probably the best-known theme of his laboratory is the exploration and commercial development of tyrosine-derived resorbable polymers, one of which is now used in an FDA-approved medical device that has so far been implanted in over 30,000 patients. He holds the position of Board of Governors Professor of Chemistry in the Department of Chemistry and Chemical Biology at Rutgers University, serves as the director of one of the two branches of the Armed Forces Institute of Regenerative Medicine (AFIRM), and is the founding director of the New Jersey Center for Biomaterials.
Kohn was born in Germany a few years after World War II. He grew up in Munich, which he describes as “one of the most wonderful cities” in Germany. He obtained his Ph.D. in chemistry and biophysics in 1983 from the Weizmann Institute of Science in Israel, working on the immobilization of enzymes and the development of chemical reactions that allow the attachment of biologically active ligands to polysaccharide beads. Kohn recalls: “My thesis adviser was Dr. Meir Wilchek. Dr. Wilchek is an extremely bright and productive scientist. He not only instilled the love of science in me, he truly influenced my life.”
Kohn was fortunate to have had another great scientist as a mentor: after completing his Ph.D., Kohn joined the laboratory of Prof. Robert Langer at MIT in 1983. At that time, he was one of the first postdocs in the Langer lab, and he joined just when Prof. Langer’s laboratory was about to make some major breakthroughs in drug delivery and tissue engineering. This was an extremely productive time but also a seminal experience that undoubtedly contributed to Kohn’s own career development. He advises young scientists to be very picky when choosing a laboratory for postdoctoral training.
Q Can you share some of your memories from the time you were a postdoc in Prof. Langer’s laboratory at MIT?
A I read with great interest the recently published interview with Allan Hoffman (CRS Newsletter 28[6], 2011), who described what life was like at MIT in the 1950s. That’s about 30 years before my time, but it is easy for me to point out some common experiences: It was intense! We worked extremely hard. And it almost seems that I could have saved myself the rent for my appartment—I spent so much time in the lab. But perhaps my most important memories relate to how stimulating and exciting my postdoctoral experience really was. I had no clue, back at that time, that I was witnessing history in the making. I met a lot of young, exciting people, all more or less at the beginning of their careers. My friendship with Nicholas Peppas (a former CRS president) started when I met him during one of his visits to Bob Langer’s lab. Likewise, I meet Jorge Heller, the inventor of poly(ortho esters); Henry Brem, the brain surgeon who helped commercialize the first biodegradable drug delivery system; and Howard Rosen, who became the president of the ALZA division of Johnson & Johnson. Some of my peers were Mark Salzmann (today at Yale University), Kam Leong (today at Duke University), Elazar Edelman (today at Harvard/MIT), Margaret Wheatley (today at Drexel University), Edith Mathiowitz (today at Brown University), Ronald Siegel (today at the University of Minnesota), and many others. I am sure that many readers of this interview will instantaneously recognize these names. I think that, as a group, these early “lab rats” from the Langer lab have changed the fields of drug delivery and tissue engineering, and it was a unique and unforgettable experience to be part of this group back in the 1980s.
Just to add one fun memory: I met John Godfrey, the inventor of zinc logenzes, which later became a huge commerical success and are commonly known as Cold-Eeze. John had just cooked up his first zinc logenzes in his kitchen (see http://pabook.libraries.psu.edu/palitmap/ColdEEZE.html) and wanted me to taste them and to help him commercialize this product.
Q You mentioned that you’ve really had four jobs while working at Rutgers for 28 years. Can you elaborate?
A I can see four very distinct stages in my career development. After my postdoctoral training at MIT, I started as an assistant professor at Rutgers in 1986. Being a professor was my first “real” job.
In 1992, I started to develop the New Jersey Center for Biomaterials (NJCBM), and I became an administrator of a major collaborative team of scientists. In my experience, there are huge differences between being an individual faculty member and being the leader of a collaborative team of scientists. I regard my directorship of the NJCBM as my second job.
Then, for one full decade, starting approximately in 1999, I became an entrepreneur. This was my third job. I founded start-up companies, participated in commercial fund-raising efforts, and became an expert in the pros and cons of academic technology transfer and commercialization.
Finally, for the last five years, I’ve served as one of the two civilian directors of the Armed Forces Institute of Regenerative Medicine (AFIRM). In this capacity, I was the principal investigator of a national research team of over 100 scientists in more than 20 institutions, a team that received about $60 million in research and clinical trial funding from the U.S. Department of Defense. I regard this as my fourth job, distinctly different from anything I had ever done before.
Q Let’s talk a bit more about your first job, being a professor. What insights can you give to our readers?
A When I joined Rutgers as an assistant professor, 100% of my effort was devoted to teaching, science, and service. This is what professors are doing. For me, teaching involved standing in front of some very large classes, as the Rutgers Chemistry Department has to teach over 9,000 students annually. I remember having over 400 students in one of the organic chemistry classes. Four hundred people looking at the professor can be an exciting or a frightening experience, depending on one’s attitude toward lecturing. Some of my students were about the same age I was—therefore, gaining control over the class and motivating the students to learn chemical formulas was a really interesting challenge. Here’s a piece of advice for the younger readers thinking about an academic career: it is not likely that you’ll get a lot of help from your senior faculty colleagues. Standing in front of a large class is a very personal learning experience. I certainly made my share of beginner’s mistakes, but over time, I also gained confidence in addressing large crowds.
Then, there is the science component. Starting a lab is a wonderful opportunity. My chairman handed me a $250,000 check, smiled, and said jokingly, “Spend it wisely—I’ll see you again, once you have the Nobel Prize.” Then he walked out, and I was left with a huge amount of money to spend and a 500 square foot empty room. My next challenge was to find good students, define projects, and hunt for grant support.
Here, I think, is a key difference between my experience and the experience of younger faculty: back in my time, the national funding rate was still over 30%. That means, on average 1 in 3 grant proposals to the NIH or NSF was actually funded. Today the proportion of funded proposals is apparently closer to 10%—and the grants being awarded are much smaller (in real dollars) than the awards I got back in the 1980s. Therefore, my experience is probably no longer typical for younger faculty: my very first proposal was funded, and within 9 months of starting at Rutgers, I was able to supplement my shrinking “start-up funds” with my first independently funded project. I remember this moment of getting a call from the program director at the NIH telling me that my proposal would be funded. I still didn’t have an office, and the call came in on a payphone in the hallway. But, these minor details not withstanding, the sense of pride, satisfaction, and joy was truly overwhelming.
The third component in a professor’s portfolio of activities is “service.” Faculty are supposed to sit on various governance committees, evaluate other people’s work as part of the peer review system, and do some general societal good. I remember that all of these activities were very rewarding, and I always felt that I gained a lot in terms of experience and exposure through these service activities.
Q Aren’t you still a professor? Have there really been major changes in your life?
A Yes, I am definitely still a professor, but I can really differentiate four phases of my career development. Therefore, what I call my “four jobs” are really four different levels of professional development while I continued to work at Rutgers.
A big change came when Dr. Stanley Bergen, the president of the neighboring medical school (University of Medicine and Dentistry of New Jersey, UMDNJ), called one day and asked me to work with him on the establishment of a biomaterials center in New Jersey. I agreed, not exactly knowing how significantly this decision would affect my life. A few weeks later a $300,000 check from UMDNJ arrived with the requirement to start a biomaterials program. Overnight, I turned from a professor to an administrator, from someone who begs others for research funding to someone who has research money to give out. I define my second job as my directorship at the New Jersey Center for Biomaterials, which started in 1992. My life really changed. I’ll just mention a few amusing tidbits: I used to be late for meetings. Jokingly, like most professors, I thought that 15 minutes late is within the academic norm. Before 1992, it never mattered that I was late, since the meetings usually started just fine without me. But that changed in 1992: suddenly, everyone would wait for me to show up. Now, my being late became very noticeable, and I had to change my habits. Also, people took me much more seriously. As a professor, I used to joke that so few students would pay attention to what I was saying in class that it really didn’t matter if I occasionally misspoke. Once in a leadership position, I learned very quickly that I had to be very careful about what I said, because people would actually listen. And finally, the amounts of money I was responsible for dramatically increased. As a professor, one usually deals with support for a single laboratory that is measured in the hundreds of thousands of dollars. As a center director, my first successful grant was a huge $3.6 million. Over time, the New Jersey Center for Biomaterials developed a highly visible and successful program of academic and industrial research. But, on the downside, I ended up spending less time in the laboratory. Therefore, my advice for younger scientists is to avoid taking on highly demanding administrative positions too early in one’s scientific career.
Perhaps the most significant insight from my “second job” was the realization of how difficult it really is to get collaboration and cooperation from individual faculty. Faculty have to spend so much time looking for funding for their own laboratories that it is really difficult for most professors to find value in being a smaller player in a larger enterprise. In addition, the rules of academic recognition and professional standing tend to favor faculty members who are principal investigators of many small grants over faculty who are making huge contributions as collaborators on a larger team. Since finding solutions for our major societal and scientific challenges requires large, interdisciplinary, and often interinstitutional teams of many great minds, the old rules of academia are not fully in line with the needs of modern science.
Q As you developed the New Jersey Center for Biomaterials, what role was played by entrepreneurship and interactions with industry?
|
|
|
Lux Biosciences and the Kohn Lab jointly developed an |
A This is a very good question, and one I actually love to respond to. I always felt that there is an unspoken societal contract between the scientific community and the taxpayers: taxpayers support our work, and in return we not only generate knowledge but also contribute to enhancing the life and health of all people. I feel that we scientists are very good at generating knowledge, but it takes an extra effort to translate this knowledge into products that enhance the lives of people. I feel that too few scientists make this extra effort.
My advice for young assistant professors is to focus initially on developing their scientific careers and building a body of scientific work and patents. But, at some point, when a “critical mass” of intellectual property has been generated, faculty should start thinking about commercialization.
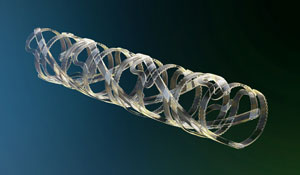
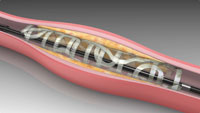
In my case, by 1996, I had about 10 issued U.S. patents. That’s when I started to think seriously about industry. I had participated in founding a first start-up company, which promptly failed. Rather than giving up and being discouraged, the failure of my first start-up company provided me with the expertise to try again. And this time it worked: my second start-up company was TYRX, a New Jersey based company that developed drug-eluting coatings for a series of innovative product concepts. Today, the products made by TYRX are used by over 30,000 patients. Quickly thereafter, I helped Rutgers enter into additional licensing agreements with several other companies. Here, I would like to highlight REVA Medical (a coronary stent company in San Diego, CA), Lux Biosciences (a company developing ophthalmic drug delivery systems in Jersey City, NJ), and finally, Trident Biomedical (a company developing orthopedic implants in Bridgewater, NJ).
|
|
|
|
Drug-eluting, fully degradable coronary stent made |
|
One thing is clear: these entrepreneurial activities are extremely time and energy consuming. Today I fully appreciate why so few faculty are willing to go down this road—and I do respect their decision not to engage in serious commercialization activities.
For me, dealing with four companies was a difficult learning experience. There is a constant need to balance one’s limited time, to avoid legal pitfalls, and to stay clear of complex conflict-of-interest regulations. And, while there are books and the occasional workshop, it is impossible to really learn how to manage academia-industry interactions without practical experience. Therefore, I regard the decade from about 1995 to 2005 as my third job.
Q In hindsight, do you believe that it was really worthwhile to engage in technology transfer and commercialization?
I want to make sure that I leave you with the notion that all of this extra effort in the end pays off in many ways. TYRX was able to bring two products to the market: one is an antimicrobial sleeve to prevent devastating (and sometimes fatal) infections among recipients of pacemakers and other coronary assist devices. So far, about 30,000 patients have been implanted with this device, and based on the significant drop in the infection rate seen in these patients, the polymer we developed and licensed to TYRX has already saved many lives. I regard this as my most important achievement. I am convinced that many academic researchers, from students to postdocs and faculty, are seriously thinking about commercializing their inventions, and I hope that the path toward commercialization will become easier over time.
Q I assume that your fourth job was being involved with the Department of Defense and the Armed Forces Institute of Regenerative Medicine (AFIRM)?
A You are correct. It was a huge step when we were awarded about $60 million to guide the work of a group of over 100 scientists in 15 major academic institutions and several
|
|
|
A service member providing first response treatment. |
companies. Our vision was to create regenerative therapies that can alleviate the human pain and suffering caused by crude bombs, often called improvised explosive devices (IEDs). Our key mission was to focus on the treatment of burn injuries, injuries to the musculoskeletal system, and injuries to the face. The Army asked us to develop therapies that can restore the body’s form and function after massive injuries. Many people are not aware that military doctors had found ways to save the lives of many seriously injured war-fighters. As a consequence of highly efficient rescue operations, the mortality rate of the wars in Iraq and Afghanistan was the lowest of any war so far. But, on the other hand, our ability to save lives on the battlefield had outgrown our ability to deal with the horrific injuries sustained by the survivors. There are about 5,000 veterans of Iraq and Afghanistan who have lost arms and/or legs or who are seriously disfigured. AFIRM was created to find medical solutions for these most seriously injured members of our armed forces. In addressing this need, drug delivery, tissue engineering, biomaterials science, cell and stem cell biology, and clinical practice were combined to make a number of major breakthroughs.
As one of the civilian directors of AFIRM, I had a glimpse into the future of science, in terms of how scientific work may be solicited by the government and how teams may be organized and managed. I think the term “megascience” is sometimes used to describe the establishment of very large collaborative teams that address major societal problems. I really believe that government support for individual investigators will shrink over the next few years and that we will see more AFIRM-like efforts that encourage a wide range of experts to collaborate toward a major common goal.
Q Could you focus on the interests of CRS members and tell us what aspects of drug delivery were important in your AFIRM work?
A Regenerative therapies rely in part on the release of biologically active agents, either systemically or locally. A good example are the bone morphogenic proteins BMP-2 and BMP-7, which are powerful activators of bone regeneration. Other growth factors were used to support the regeneration of cartilage. A project that proceeded in our lab was addressing the serious problem that partial thickness (2nd degree) burns can progress after the injury occurred to full thickness (3rd degree) burns. When that happens, medical treatment becomes more complicated and costly, and the formation of scars can lead to lifelong disability. Together with Dr. Richard Clark, a leading burn injury expert from Stony Brook University, we worked on developing a burn wound dressing that is impregnated with P12, a proprietary peptide that can prevent burn injury progression. Formulating an appropriate release system for P12 as part of a wound dressing turned out to be a major challenge. We finally settled on incorporating P12 into a nanofiber mat made of a uniquely designed polymer that provided the required seven-day release profile for P12. These are just a few examples of the important role drug delivery technologies play in the implementation of regenerative therapies.
Q Finally, can you tell us about past and current research work in your laboratory?
A I am very excited about the successful development of a new “discovery paradigm” for revolutionary biomaterials using combinatorial and computational methods to optimize the
|
|
|
Das Bolikal (left) and Joachim Kohn (right) in the |
composition and properties of biomaterials for specific applications. Briefly, we started to look at the way the pharmaceutical industry is discovering new lead compounds for drug development. We realized that new biomaterials were usually discovered (or invented) based on serendipity rather than as a consequence of a rational design approach. That meant there was a lack of bioactive polymers specifically designed for advanced applications such as tissue engineering and drug delivery. As a first demonstration of the utility of this approach, we applied a systematic design paradigm to discover an optimized polymer for use in a fully degradable cardiovascular stent. Our polymer enabled a new stent design, which is now being tested in clinical trials in Germany and Brazil. Additional examples of our use of a combinatorial biomaterials design approach are the development of optimized polymers for Lux Biosciences (ophthalmic applications) and for Trident Biomedical (orthopedic applications).
Much of our recent work builds upon our invention of pseudo-poly(amino acids), amino acid–derived, degradable polymers in which individual amino acids are linked by nonamide bonds. While most conventional poly(amino acids) have poor engineering properties, pseudo-poly(amino acids) can be designed to exhibit outstanding engineering and physicomechanical properties, as well as a high degree of biocompatibility. For example, tyrosine-derived polycarbonates were shown to be promising materials for implantable drug delivery systems and orthopedic implants, copolymers of the natural amino acid L-lysine and poly(ethylene glycol) were successfully used as drug carriers, and polymeric conjugates of cis-hydroxyproline were prepared and found to exhibit powerful antifibrotic activity. These insights allowed us to develop computational models that can predict many important polymer properties, based simply upon the knowledge of the chemical composition of the polymer.
|
|
|
Kohn Lab members crowd the staircase in their new Life Sciences Building |
Q To conclude, can you share with us your connections with CRS?
A CRS was the first major scientific society I joined as a postdoc back in 1984. I have vivid memories of my very first international travel to give my first public lecture at the CRS meeting in Geneva, Switzerland, in 1985. At that time, I made my first professional connections, and some of these contacts became part of my professional life for years. CRS was definitely an important part of my professional development. We didn’t use a lot of e-mail prior to 1990, and at that time the personal contact with colleagues, friends, and scientific competitors was very important. The CRS Annual Meetings were the highlights of my travel calendar. Then, in 1992, I was the recipient of the CRS Young Investigator Award—a very important event in my professional life, as it came right around the time I was coming up for the the critical tenure decision and promotion to associate professor. I still display my CRS award prominently on my desk.
In spite of the advance of e-mail, internet, and smart phones, the need for personal interactions remains as strong as ever; therefore, I am sure that professional societies like CRS will continue to provide a vital service to the scientifc community. I also hope that CRS continues to occupy a central role in the professional development of young scientists today. My final thought is directed toward the leadership of CRS: I hope that CRS will continue to provide mentorship opportunities and other opportunities to grow to our younger members.