By Jake Barralet and Marc Thibault, Orthopaedics Division, Department of Surgery, Montreal General Hospital, Faculty of Medicine, McGill University, Montreal, Quebec, Canada
At the beginning of 2012, the world of controlled release was changed forever with the fascinating report in the journal Science Translational Medicine of the first successful human trial of wirelessly delivered multiple doses of human parathyroid hormone fragment [hPTH(1-34)]. Bob Farra will be speaking at the 2013 CRS Canadian Local Chapter meeting in Vancouver on “Enabling Remote Medicine: Implantable, Wirelessly Controlled Microchip Drug Delivery.”
 Robert Farra served as vice president of research and development for MicroCHIPS from 2007 to 2011 before becoming its president and chief operating officer. Prior to joining MicroCHIPS, he served as vice president of engineering and manufacturing for Abiomed, a global leader in technologies that assist or replace the pumping function of failing hearts. He also held senior leadership positions at Accellent Endoscopy and Arthur D. Little, Inc., where he led new product development efforts for Fortune 500 firms as well as start-ups.
Robert Farra served as vice president of research and development for MicroCHIPS from 2007 to 2011 before becoming its president and chief operating officer. Prior to joining MicroCHIPS, he served as vice president of engineering and manufacturing for Abiomed, a global leader in technologies that assist or replace the pumping function of failing hearts. He also held senior leadership positions at Accellent Endoscopy and Arthur D. Little, Inc., where he led new product development efforts for Fortune 500 firms as well as start-ups.
Farra and coauthors caught the world’s attention last year with their paper (Farra et al. 2012) describing the first human trial of a subcutaneously implanted wireless drug delivery microchip. The device is based on proprietary reservoir arrays, each of which has 20 platinum-titanium sealed apertures that can be opened electrically. This technology represents an important shift in drug delivery, tackling the challenging issues of poor compliance in complex drug regimens typical of chronic pathologies such as diabetes and osteoporosis. We caught up with Bob to discuss MicroCHIPS’s technology and latest progress in this field.
Q Please explain a little more how the MicroCHIPS device works. We understand that there is a computer-based programmer operating in the Medical Implant Communication Service band, but how does this work in practice? Is the dosing schedule preloaded into the device, or does the device need to be instructed to release a dose in real time; are there issues with distance between the patient and the controller; and so on?
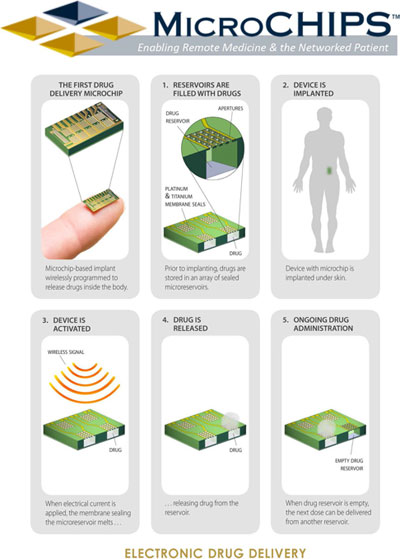
A The microchip-based implant has on its surface a microchip that is in direct contact with the subcutaneous tissue. The microchip has discrete doses of drug, and each dose is hermetically sealed. Within the implant there are control electronics including radio frequency communications, a real-time clock to ensure accurate timing for release of each dose, a microcontroller to enable control of all necessary functions, and custom circuitry that is electrically connected to each dose to allow independent addressing of each dose at any time or in any sequence. An external device communicates with the implanted device through radio frequency. The external device can provide instructions to release a dose instantly, or at a future time, or instructions to release any number of doses at specific time points in the future. The external controller can be a cellular phone, a tablet, or a custom transceiver connected to a personal computer (similar to the device we used in the clinical trial). The distance between the body and the external controller must be within 3 m to establish communication. The benefits of low transmission range are low power consumption and prevention of unwanted communications attempts.
|
|
|
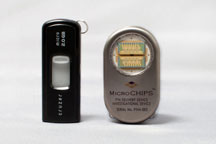
Wireless controller (left) and implantable delivery system (right). |
Q Besides compliance issues, what is the major problem your device addresses?
A The microchip technology provides extreme flexibility in delivering drugs. Scheduled, automated drug delivery addresses compliance issues and ensures patients receive their medication as required. The patient receives optimum therapy, which results in improved outcomes and, consequently, reduced healthcare costs. In addition, different dose sizes can be delivered, providing benefits that address difficult dosing regimens such as weaning patients on or off of certain medications as well as titrating the appropriate dose in response to the patient’s physiological changes. The microchip can also contain multiple drugs customized for each patient’s needs, and these drugs can be released per a customized schedule.
Other benefits include the ability to provide automated and timely dosing for patients that lead an active lifestyle, freeing them from carrying syringes and medication to and from their destinations.
Children can benefit from this technology by providing medication to treat, for example, an anaphylaxis response. Dosing can be remotely triggered by a parent, teacher, or clinician as required in an emergency situation. Similarly, military applications include the wireless triggering of medication to treat trauma in remote locations until medical attention becomes available.
Another application is the delivery of pain management medication for end-of-life situations. Patients can spend their last few months with their family, and their family members don’t need to worry about providing proper dosing, since the dosing schedule can be programmed in advance or modified by a clinician from a wireless, cellular device.
 |
|
Schematic showing operations of MicroCHIPS's technology |
Q Is this device mainly aimed at older patients, and do you think patients need to be comfortable with technology in the first place to accept fitting of your device?
As I mentioned above, children, active adults, and military or service personnel can benefit from this technology. Elderly patients, and for that matter, all patients, don’t need to be knowledgeable with computers, programming, or smart phone use. The clinicians or caregivers are able to program the implant; the patient is not required to take any action or remember to do anything.
Q How do you deal with security issues such as hacking that can happen with a wireless device? Your paper noted that sometimes not all 20 apertures opened but that pharmacokinetic profile was not affected until failure was above 25%. How do you avoid accidental overdosing and multiple reservoirs opening? What electronic checks are performed prior to implantation?
A We have taken significant measures to ensure safety, reliability, and security. Each of these requires a separate design approach. Regarding safety, our implant has multiple hardware and software checks and back-up systems. For example, we limit the electronic current available such that only one reservoir can open at a time. Should a hardware failure occur, the resulting electronic circuit will be an open circuit, thus disabling the release of a drug. We also have designed software algorithms that keep track of when the last dose was released and require a set time period to elapse prior to enabling the release of the next dose.
Regarding reliability, there are multiple apertures per reservoir to release the drug. The devices used in the clinical trial had some membrane fabrication inconsistencies that have been corrected by the manufacturer. These inconsistencies should no longer be present in future builds. We have also enhanced the number of tests performed during manufacturing, postmanufacturing, and prior to implantation to ensure all implanted devices are fully functional. Reliability is expected to be high based on our tests and given that there are no mechanical parts to wear out.
Regarding security, we have assigned a unique serial number to each implant and incorporated a proprietary communication protocol to ensure that no unintentional communication is possible.
Q From the images, one might imagine that most of the volume of the device is the battery. In the future, do you think the device could use an induction coil to reduce volume? How else could the device be further miniaturised?
A We have made significant progress since our clinical trial. We have conceived an improved design that increases the number of reservoirs by a factor of 10, increases the dose volume by a factor of two, and reduces the overall device volume by a factor of 5. These three changes equate to a 100-fold improvement in the drug to device volume ratio. We are not yet at liberty to discuss how we have achieved such significant design improvements.
Q Do you think the scar left after use is a barrier to acceptance?
A The implant and explant scar is a function of several factors, including the size of the implant (which we have significantly reduced) and possibly the surgeon’s technique and the patient’s healing response. Our new, smaller implant also has a longer use duration, further improving acceptability.
Q In your paper you waited nearly two months to allow the fibrous capsule to form prior to starting dosing. Was this to create steady conditions for reproducible delivery or to provide a better test of safety?
A Based on previous research (Anderson 2001), we believe that the fibrous capsule should be fully formed within three to four weeks. However, since this was the first use of this technology in humans, we wanted to assess the pharmacokinetics under steady state capsule conditions. We wanted to evaluate the drug/microchip release kinetics as well as to determine what effects there are, if any, from the fibrous capsule. The only way to have made this assessment was to release the drug after the capsule was fully formed.
Q You lyophilised the parathyroid hormone fragment in the reservoirs. Is this the only way to load the microchip?
A We have developed processes and equipment that allow us to fill the microchip reservoirs with a liquid, solid, gas, or a combination of these.
Q Can liquid formulations be delivered by this device?
A Yes, liquids can be delivered, and we do not expect it to be necessary to make design or manufacturing changes to accommodate liquid formulation and delivery.
Q What other conditions besides osteoporosis do you envision?
A Some of the other indications we have been investigating include drug delivery to treat multiple sclerosis, reduce pain, as well as other hormone delivery for various applications. We are considering both human and animal applications.
Q Could the microchips ever be refillable without being explanted?
A There may be possible design configurations that lend themselves to some level of refilling. However, our near-term focus is on design applications that are not refillable.
Q You mention the intriguing possibility of using sensors to provide closed-loop feedback for delivery. One thinks immediately of diabetes. Could you expand a little more on this and tell us what progress there is?
A This technology also lends itself nicely to storing a matrix of sensors and exposing each sensor when needed. We have designed and successfully prototyped in animals continuous glucose sensors. We believe it’s feasible to combine on the same implant both sensors and drug delivery microchips. We can sense if a patient is trending toward a hypoglycemic condition. If the trend continues, we can release glucagon to reverse that trend. We have also identified other “rescue” type applications that provide drug delivery in response to physiological changes.
Q Upon application of a current, the metallic film balls up to open the aperture. I believe platinum can cause sensitization. What is the fate of the metal film following ablation, and how did you arrive at this composition?
A When we apply the appropriate electric current through the membranes covering each reservoir, the membranes melt and resolidify at the perimeter of each aperture. One of the benefits of the resulting bead is that this feature is atraumatic. We have studied and confirmed this behavior analytically and under in vitro and in vivo conditions. We have also conducted in vivo and in vitrobiocompatibility tests out to 52 weeks per ISO 10993 and observed no differences between our implants and control devices. Also, note that the mass of platinum is extremely small (less than 0.25 mg per reservoir).
Q In a field that bridges pharma, IT, and engineering, what is the single most important expertise you want at your company to take this technology to the next level?
A As you suggest, our technology requires skill sets in multiple technical fields. We will continue to require skills in microchip design, miniaturization of electronics, wireless communication, and medical implant design. Our perspective is that there are several excellent pharmaceutical companies that continuously identify new drug molecules. We will be partnering with these pharmaceutical companies to package and precisely deliver their drugs to achieve optimum therapeutic benefit.
Q The field of degradable electronics is quite hot. Do you foresee a day when microchips may not need to be removed?
A Absolutely. We will continue integrating and miniaturizing our implant. The next step in the future will be to incorporate biodegradable components.
Q What new enabling technologies are you excited about?
A We are fortunate to benefit from advancements in microelectromechanical systems fabrication and electronic miniaturization. These will allow us to approach much higher drug to device volume ratios and will continue to transform our implant into a truly minimally invasive and programmable drug delivery device. In addition, cellular technology will allow us to integrate our implant with physician, caregiver, and healthcare services to provide integration with diagnostic and feedback controlled therapy.
 |
|
PTH Chip |
References
Farra, R, Sheppard, NF, McCabe, L, Neer, RM, Anderson, JM, Santini, JT, Jr., Cima, MJ, Langer, R. First-in-human testing of a wirelessly controlled drug delivery microchip, Sci. Transl. Med. 4(122): 122ra21 (2012). DOI: 10.1126/scitranslmed.3003276
Anderson, JM. Biological responses to materials, Annu. Rev. Mater. Res. 31: 81-110 (2001).