|
By Vicki Ronaldson Improving the bioavailability of poorly water soluble drugs has long been a necessary preoccupation of the pharmaceutical industry. And it’s a problem Texas-based contract development company DisperSol Technologies knows a thing or two about solving. Launched in 2008 to exploit technology developed in collaboration with the University of Texas at Austin, the company boasts an impressive management team and has successfully commercialized its proprietary KinetiSol® technology, an innovative fusion process for the preparation of solid dispersions. The technology, adapted from a commercial plastics manufacturing process and making use of heat generated from shear and friction, has been the subject of numerous publications, most recently in the International Journal of Pharmaceutics. I got the “inside track” on this technology from Dr. Dave Miller, Vice President of R&D at DisperSol Technologies. Q: Many thanks for agreeing to talk to us, Dave. You’ve certainly been busy on the publication front; the KinetiSol process clearly encompasses a number of innovations. Improving bioavailability through enhancing aqueous solubility is something of a “holy grail” of drug delivery, and it’s clearly a field with which you’re very familiar, having recently coedited Formulating Poorly Water Soluble Drugs(Springer and AAPS Press, 2011). What’s different about KinetiSol? KinetiSol is a fusion-based process for the manufacture of amorphous solid dispersion systems, and as such, offers numerous benefits of nonsolvent processing. What makes KinetiSol a unique thermal process is its very brief processing times, its high-energy mixing mechanism, and its ability to process highly viscous compositions. KinetiSol processing times are typically under 20 seconds, and elevated temperatures are maintained for less than five seconds. This enables thermal processing of heat-sensitive APIs and excipients without degradation by reducing cumulative thermal stress. The high-energy mixing of KinetiSol promotes rapid solubilization kinetics and enables formation of amorphous dispersions well below the melt transition of the API. Amorphous solid dispersions with high drug loadings can be achieved with high melting compounds in a variety of polymeric carriers, even those prone to thermal decomposition. The high-energy mixing mechanism coupled with the ability to process highly viscous polymers enables intimate mixing of APIs with nonthermoplastic and high-molecular-weight polymers without the use of plasticizers. We have published papers demonstrating single-phase, physically stable systems by KinetiSol with polymers that are difficult or impossible to thermally process without the use of processing aids.
Q: KinetiSol has its roots in a collaborative research effort carried out at the University of Texas at Austin. How did this research evolve into a commercial entity? Was it premeditated or a serendipitous discovery?
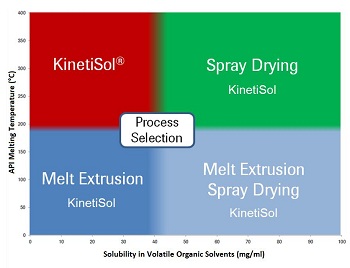
KinetiSol was something of a serendipitous discovery. I was conducting melt extrusion research at the University of Texas when I met Chris Brough, who is now DisperSol’s Vice President of Technology. Chris is a pioneer and inventor of the process in the plastics industry. As I came to understand his process and he became acquainted with my research, we began to realize the potential of the process as a pharmaceutical manufacturing operation. Chris and I conducted the first KinetiSol trials on commercial-scale plastics processing equipment with results exceeding expectations. Based on the success of those initial trials, work began to adapt the process to pharmaceutical manufacturing and to scale down the equipment to accommodate laboratory environments. Also at that time, patent applications were filed jointly by DisperSol and the University of Texas. While DisperSol owns patents claiming the process and equipment, the IP for pharmaceutical applications is jointly owned by the university and DisperSol. DisperSol has an exclusive license from the university to commercialize the process, and we are currently working with several major pharmaceutical companies and excipient manufacturers toward applications of KinetiSol with their compounds and products. Q: What were the major technical challenges in adapting a plastics manufacturing technique to work for pharmaceutical manufacturing? And what regulatory requirements was the equipment subject to? The path to adapting KinetiSol from plastics processing to cGMP pharmaceutical manufacturing closely mirrored that of hot-melt extrusion. The critical aspects were to ensure a high degree of cleanliness, process reproducibility, and thorough documentation. All product contact parts were made from hardened stainless steel that is not reactive, additive, or absorptive, in accordance with the Code of Federal Regulations (21 CFR 211). Other key modifications versus the plastics process included ground and polished welding seams, calibration for all process parameters, the use of NSF certified oils and lubricants, the use of USDA-approved epoxy for non-product-contact parts, and validation of computer-based controls to support FDA review. The KinetiSol process was designed for pharmaceutical applications with the GMP philosophy in mind, that is, reproducibility, cleanability, and validation. Q: So what are the key problems/drug types you see KinetSol® solving? Where KinetiSol adds the most value to the field of amorphous solid dispersion processing is with thermally labile APIs and excipients, high-melting-point compounds, and highly viscous/high-molecular-weight polymers. Each of these represents a significant limitation for melt extrusion, and hence are areas where KinetiSol is able to fulfill unmet needs. Currently, spray drying is the most frequently employed option when melt extrusion is not viable; however, spray drying can be undesirable owing to its use of organic solvents as well as higher operating and capital costs for high-volume GMP production. Additionally, we are seeing a growing subset of poorly water soluble molecules that have high melting points and are poorly soluble in volatile organic solvents. In this region of the insoluble drug space, KinetiSol may be the only viable processing option. The accompanying schematic illustrates process selection on the basis of melting point and organic solvent solubility. As you can see, KinetiSol is a preferred technology for molecules that exhibit high melting points and poor organic solubility. KinetiSol is also utilized in the other areas when thermal or solution instability issues exist or when issues related to solvent processing are a concern.
Q: Modified release is obviously essential for many formulations. Are there any limitations to the polymer types that the process can be used with? The process is compatible with all polymers commonly used in solid dispersion systems, including HPMCAS, copovidone, povidone, HPMC, methacrylic acid copolymers, polyethylene glycol, poloxamer, and Soluplus. Furthermore, KinetiSol is able to process high-molecular-weight grades of HPMC and povidone. We are also working on applications employing cross-linked materials. High-molecular-weight and cross-linked polymers are extremely difficult, if not impossible, to process by melt extrusion and also present serious viscosity issues for spray drying. Typically, plasticizers are required for melt extrusion with these polymers to the detriment of product stability. KinetiSol is able to process these polymeric materials to form single-phase systems without plasticizers. The variety of excipient materials that can be processed by KinetiSol and incorporated into solid dispersion systems is presenting opportunities for unique drug-polymer combinations that cannot be achieved by other processes. You bring up a good point regarding modified release. Our most effective compositions are amorphous systems that utilize anionic (enteric) polymers. These carriers promote targeted supersaturation to the proximal small intestine, which has been proven to optimize oral absorption for numerous insoluble compounds. However, these polymers tend to have onsets of thermal degradation below the melting points of many poorly water soluble drugs. KinetiSol enables production of bioavailability-enhanced compositions containing heat-sensitive anionic polymers irrespective of the drug’s melting point. Q: KinetiSol is protected by several patents, as well as a trademark on the name. IP can often be a bit of a minefield; how did you find the experience of developing the company’s IP strategy, and would you have any advice for start-ups? For a small company with a novel technology such as ours, IP protection is essential. From the outset, we teamed up with one of the largest IP law firms with extensive general IP experience and particular expertise in the pharmaceutical field. Our partnership with the University of Texas is also critical to defending our IP. I would advise other start-ups to invest heavily in strengthening their IP portfolio and to develop strategies for defending against infringement. Q: Now that KinetiSol has gained commercial traction, what’s next in terms of R&D? And how important is maintaining an R&D pipeline to the company? It is DisperSol’s mission to enable the development and commercialization of new medicinal therapies from promising compounds that would otherwise be unattainable due to solubility and processing issues. We are currently working with several large pharmaceutical companies, conducting KinetiSol feasibility studies with their most challenging compounds. Successful feasibility studies then transition into manufacturing for preclinical and early phase clinical studies. As these programs progress, they will flow into our high-volume manufacturing operations for late-phase clinical trials and ultimately commercial production. Our R&D group is also busy with internal development projects. We have identified compounds with therapeutic potentials that have not been fully realized owing to solubility and processing issues. We are applying KinetiSol and advanced formulation design to these molecules to enhance delivery and improve therapeutic outcomes. The ultimate goals for these projects are to enable more effective therapies for improved patient outcomes and to realize new indications for existing drugs. We are also developing new drug delivery applications for KinetiSol. We are continuing to improve upon the solubility enhancement aspects of the technology as well as evaluating innovative controlled release applications. Some of this work is being supported by and conducted in collaboration with large excipient companies. In summary, our R&D pipeline is quite full with a variety of projects. The quantity and breadth of projects is critical to the long-term success of DisperSol Technologies.
Website: www.dispersoltech.com
Recent Publications
Miller, DA, DiNunzio, JC, Hughey, JR, Williams, RO, 3rd, McGinity, JW. KinetiSol®: A new processing paradigm for amorphous solid dispersion systems, Drug Dev. Delivery (in press, October 2012).
Hughey, JR, Keen, JM, Miller, DA, Brough, C, McGinity, JW. Preparation of viscous solid dispersion systems 1 by hot-melt extrusion and KinetiSol® dispersing: Polymer screening and thermal stability, Int. J. Pharm. (in press, published online September 2012). DiNunzio, JC, Brough, C, Miller, DA, Williams, RO, 3rd, McGinity, JW. Applications of KinetiSol® dispersing for the production of plasticizer free amorphous solid dispersions, Eur. J. Pharm. Sci. 40: 179-187 (2010). See www.dispersoltech.com/publications.php for a full list of publications.
© 2012 |




 Contact:
Contact: 