It is still a pending issue for many “smart” nanocarriers endowed with a controlled and site-specific drug release mechanism to overcome the therapeutic efficacy and minimize therapy-associated side effects obtained by their older brothers, the golden benchmarked nonsensitive formulations. Motivated by current clinical development stage of enzyme-sensitive systems, in particular of phospholipase A2 (sPLA2 )-degradable formulations, we aim in the present work to revisit the potential use of sPLA2 - sensitive liposome formulations of platinum drugs, here oxaliplatin (L-OHP), and to get a deeper insight into the sPLA2 dependency of previous developed formulations, understanding the therapeutic window that is obtainable with such formulations. We report here L-OHP loaded liposomes with low or high sensitivity toward sPLA2 -triggered degradation as drug delivery nanocarriers, and we compare these slow- and fast-releasing formulations to non-sensitive nanocarriers, assessing their in vitro cytotoxicity against cultured cancer cells, as well as their efficacy and tolerability in vivo in mice bearing human tumors. A graphical abstract is presented as Figure 1.
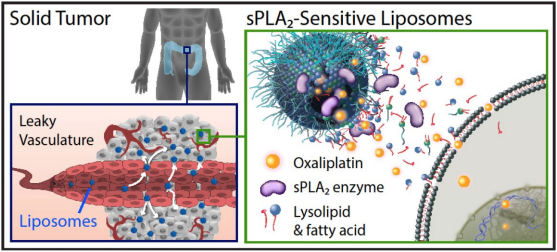 Figure 1. Graphical abstract.
Figure 1. Graphical abstract.The enzymatic activity of sPLA2 toward liposome degradation and consequently release of their cargo can be significantly modulated by the composition of the lipid bilayer and its morphological and physicochemical properties. This was demonstrated in in vitro release studies using calcein as a drug surrogate at self-quenching concentrations loaded within the different liposome carriers. We show that the molar ratio of negatively charged lipid present in the liposome membrane played a key role in the enzyme activity evaluated from calcein release profiles (Fig. 2A). In membranes composed of DPPC/DPPG/DSPEPEG2k, when 25% of the negatively charged lipid DPPG was present in the bilayer (DP(25)), only the sPLA2 from tears was able to cause partial calcein release, whereas increasing the percentage of DPPG lipid to 40% (DP(40)), the membrane became fully enzyme-degradable and calcein was completely released within 1,500 s irrespective of the source of enzyme (sPLA2 in human tears = 19.3 µg/mL; sPLA2 in cell conditioned media (CCM) = 185.4 ng/mL).1 DSC of the formulations (Fig. 2B) showed that liposomes made of 16-carbon chain lipids (DP(25) and DP(40)) have an overall main phase transition temperature (Tm) of 41 and 42°C, respectively, and were highly sensitive toward sPLA2 degradation, whereas in 18-carbon lipid membranes (DS(40)) with higher Tm of 55°C, the enzyme activity was almost abolished, which can be explained by its reduced access to the cleavable region in the membrane owing to its gel state behavior. This was evident from the time it took to reach 50% release (T50) of calcein with DP(40) liposomes (70.1 s) being >10 and >30 times faster than DP(25) liposomes (857.7 s) and DS(40) liposomes (>2,000 s), respectively (Fig. 2A, inset).
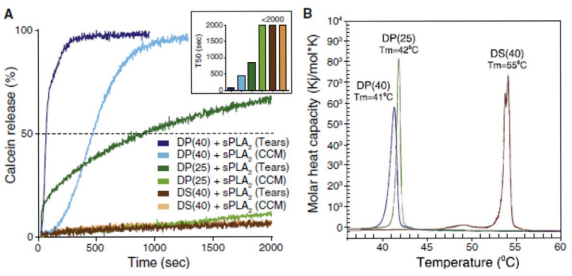 Figure 2. Calcein release profiles and phase transition diagrams of liposome formulations. (A) In vitro sPLA2 -dependent release kinetics of calcein from liposomal formulations incubated at 37°C. Human tear fluid and cell conditioned media (CCM) from human Colo205 cancer cells were used as enzyme sources of sPLA2 . All results are expressed relative to 100% release observed after addition of Triton X-100. Results are representative of at least three experiments. Inset shows the time-intersect for the individual formulations reaching 50% release (T50) in seconds. (B) DSC endotherms highlighting the main phase transition temperature (Tm) of the different formulations. Abbreviations: DP(25) = DPPC/DPPG/ DSPE-PEG2k (70:25:5 mol%); DP(40) = DPPC/DPPG/DSPE-PEG2k (55:40:5 mol%); and DS(40) = DSPC/DSPG/DSPE-PEG2k (55:40:5 mol%).
Figure 2. Calcein release profiles and phase transition diagrams of liposome formulations. (A) In vitro sPLA2 -dependent release kinetics of calcein from liposomal formulations incubated at 37°C. Human tear fluid and cell conditioned media (CCM) from human Colo205 cancer cells were used as enzyme sources of sPLA2 . All results are expressed relative to 100% release observed after addition of Triton X-100. Results are representative of at least three experiments. Inset shows the time-intersect for the individual formulations reaching 50% release (T50) in seconds. (B) DSC endotherms highlighting the main phase transition temperature (Tm) of the different formulations. Abbreviations: DP(25) = DPPC/DPPG/ DSPE-PEG2k (70:25:5 mol%); DP(40) = DPPC/DPPG/DSPE-PEG2k (55:40:5 mol%); and DS(40) = DSPC/DSPG/DSPE-PEG2k (55:40:5 mol%).Efficient sPLA2 -dependent growth inhibition of colorectal cancer cells was demonstrated in vitro. The antiproliferative capacity of free L-OHP and L-OHP loaded in sensitive DP(25) or DP(40), and in non-sensitive (stealth) formulations were tested by MTS assay in sPLA2 -secreting Colo205 cell line and in non-secreting HT-29 cells with added exogenous sPLA2 using cell CCM from Colo205 cells as a source of enzyme (Fig. 3). In concordance with the liposome sensitivity of sPLA2 shown in Figure 2A, efficient growth inhibition of sPLA2 non-secreting HT-29 cells was only observed when the liposomes became activated by exogenous sPLA2 (Fig. 3A). In both colorectal cancer cell lines sPLA2 -sensitive liposomal L-OHP were highly cytotoxic, with drug concentrations able to inhibit cell growth by 50% (IC50) around 10–12.5 μM (Fig. 3B). In addition, the degree of growth inhibition of HT-29 cells induced by the liposomes followed the sensitivity of the formulations toward sPLA2 hydrolysis (Fig. 3B). In control experiments in which HT-29 cells were treated in the absence of sPLA2 (without Colo205 CCM), the sensitive liposomes showed similar antiproliferative profiles as the non-degradable stealth formulation.1 Taken together, these findings illustrate an efficient and stable formulation of L-OHP in enzymesensitive liposomes that carry a desirable controlled release mechanism based on sPLA2 -mediated activation.
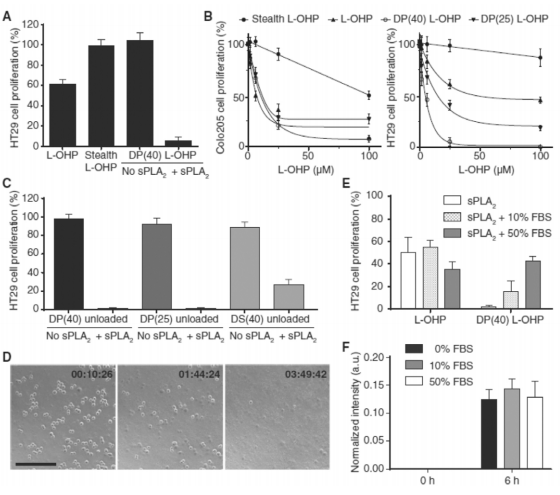 Figure 3. In vitro evaluation of the sPLA2 -sensitive concept. (A) Lack of cytotoxicity of sPLA2 -sensitive liposomes against non-sPLA2 -secreting HT-29 cells in the absence of exogenous sPLA2 shows the specificity of the concept toward sPLA2 and dependence of sPLA2 activation. (B) In vitro cytotoxicity of L-OHP in free form or encapsulated in sPLA2 -sensitive liposomes (DP(25) and DP(40)) or in non-degradable liposomes (stealth) tested against sPLA2 -secreting Colo205 cells and sPLA2 -deficient HT-29 cells in the presence of Colo205 cell conditioned medium (CCM) containing sPLA2. (C) Cytolytic activity of unloaded (without L-OHP) sPLA2 -sensitive liposomes arise from cellular lysis as a consequence of sPLA2 -driven hydrolysis and formation of permeability enhancing/membrane lysing components. (D) Time-lapse micrographs of HT-29 cells following 3 h of incubation with DP(40) in the presence of sPLA2 - proficient Colo205 CCM. (E) Inhibitory effect of sPLA2 -sensitive liposomes on HT29 cell proliferation is modulated by presence of serum components. (F) Effect of serum (10–50% FBS) on the enzymatic activity of sPLA2 . Results are triplicates from one experiment (mean ± SD) and representative of three independent experiments. Abbreviations: L-OHP = oxaliplatin; DP(25) = DPPC/DPPG/DSPE-PEG2k (70:25:5 mol%); DP(40) = DPPC/DPPG/DSPE-PEG2k (55:40:5 mol%); and DS(40) = DSPC/DSPG/DSPE-PEG2k (55:40:5 mol%); stealth = HSPC/Chol/ DSPE-PEG2k (57:38:5 mol%).
Figure 3. In vitro evaluation of the sPLA2 -sensitive concept. (A) Lack of cytotoxicity of sPLA2 -sensitive liposomes against non-sPLA2 -secreting HT-29 cells in the absence of exogenous sPLA2 shows the specificity of the concept toward sPLA2 and dependence of sPLA2 activation. (B) In vitro cytotoxicity of L-OHP in free form or encapsulated in sPLA2 -sensitive liposomes (DP(25) and DP(40)) or in non-degradable liposomes (stealth) tested against sPLA2 -secreting Colo205 cells and sPLA2 -deficient HT-29 cells in the presence of Colo205 cell conditioned medium (CCM) containing sPLA2. (C) Cytolytic activity of unloaded (without L-OHP) sPLA2 -sensitive liposomes arise from cellular lysis as a consequence of sPLA2 -driven hydrolysis and formation of permeability enhancing/membrane lysing components. (D) Time-lapse micrographs of HT-29 cells following 3 h of incubation with DP(40) in the presence of sPLA2 - proficient Colo205 CCM. (E) Inhibitory effect of sPLA2 -sensitive liposomes on HT29 cell proliferation is modulated by presence of serum components. (F) Effect of serum (10–50% FBS) on the enzymatic activity of sPLA2 . Results are triplicates from one experiment (mean ± SD) and representative of three independent experiments. Abbreviations: L-OHP = oxaliplatin; DP(25) = DPPC/DPPG/DSPE-PEG2k (70:25:5 mol%); DP(40) = DPPC/DPPG/DSPE-PEG2k (55:40:5 mol%); and DS(40) = DSPC/DSPG/DSPE-PEG2k (55:40:5 mol%); stealth = HSPC/Chol/ DSPE-PEG2k (57:38:5 mol%).Because a triggered release strategy based on sPLA2 activity is known to generate permeability-enhancing lysolipids and fatty acids, we investigated their contribution to the cytotoxic effect by measuring the growth inhibition of HT-29 cells treated with unloaded sPLA2 -sensitive liposomes with and without added sPLA2 (Fig. 3C). For HT-29 cells, both the DP(25) and DP(40) formulations were able to induce complete growth inhibition of the tumor cells at both molar ratios of the PG lipid in the membrane investigated, whereas the DS(40) formulation only induced partial growth inhibition despite the presence of 40% PG. Time-lapse micrographs of HT-29 cells during treatment with unloaded DP(40) liposomes and sPLA2 -containing CCM revealed that the observed efficient growth inhibition was in effect owing to the complete disruption of the cellular membranes (Fig. 3D). Thus, based on the evidence from the calcein release experiments and the in vitro cytotoxicity, sPLA2 - sensitive liposomes were confirmed to act as efficient prodrugs, in which their in vitro antitumor efficiency was primarily owed to the generation of cell-permeable byproducts from the action of the enzymatic hydrolysis
Serum albumin has previously been shown to bind up to five lysophospholipids per albumin molecule,2 and therefore the presence of high amounts of plasma proteins is expected to strongly modulate the permeability-enhancing properties of these components, and conversely thereby affect the effective cell lytic activity of sPLA2 -sensitive liposomal carriers as well. For this reason, to address the effect of serum on the anti-proliferative effect of sPLA2 -sensitive liposomes, HT-29 cells were treated with sPLA2 -sensitive liposomal L-OHP together with Colo205 CCM in the absence of serum or spiked with 10% (low) or 50% (high) fetal bovine serum (Fig. 3E). We demonstrate that high levels of serum proteins eliminate the cytotoxic effect of the carrier. These results support that the in vivo cytotoxic potential of generated permeability-enhancing products will not only depend on the intratumoral lipid concentration but also on the concentration of various plasma proteins present in the interstitial compartment of the cancer. Additionally, to rule out that the presence of serum affects the enzymatic activity of sPLA2 , we measured the amount of lysolipids generated after treatment of sPLA2 -sensitive liposomes with enzyme in the presence of serum using mass spectroscopy. The level of lysoPPG generated was found to be unchanged from DP(40) liposomes in the presence of serum (Fig. 3F). This demonstrates that even though components in serum can sequester the generated permeability-enhancing components from sPLA2 -sensitive liposomes, the encapsulated drug is released as sPLA2 hydrolyzes the liposomes, irrespective of the presence of serum proteins. Of course, this process is further complicated by the presence of lipase inhibitors in a clinical setting, a feature not readily captured and accounted for here by using murine and bovine serum models.
Previous findings have shown that several inbred mouse strains have an intact murine group-II sPLA2 gene, which raises the likelihood of background sPLA2 levels being present in the NMRI nude model used herein, even though the grafted tumor cells do not secrete the sPLA2 enzyme.3 We first evaluated the efficacy and tolerability of L-OHP formulated in sPLA2 -sensitive liposomes in a dose escalation study in NMRI nude mice bearing non-sPLA2 -secreting FaDu squamous carcinoma. This was done to determine the baseline of the antitumor efficacy of these particle systems in a tumor model that lacked the capacity of stimulating specific drug release and, furthermore, to establish the maximum tolerated dose including any potential unforeseen side effects occurring from premature activation by murine phospholipases. The mice received 6 i.v. doses of 4 or 8 mg/kg every 4 days, with the first dose administered upon the tumors reaching 50 mm3 in size. L-OHP formulated in DP(40) liposomes at 4 mg/kg was able to induce a minor increase in tumor growth reduction compared with mice receiving free L-OHP at equimolar doses, reaching an equivalent response rate as twice the amount of free drug injected; however, this was only a moderate effect (Fig. 4A). In turn, the low antitumor effect did not give rise to an increase in survival rates (Fig. 4B). Increasing the dose of L-OHP formulated in DP(40) liposomes to 8 mg/kg (equivalent to a lipid dose of 113 mg/kg) was poorly tolerated and led to petechial cutaneous hemorrhages in the skin of the mice,1 along with concurrent weight loss (Fig. 4C) and dehydration requiring immediate euthanasia. At this high dose, DP(40)-treated mice showed severe signs of toxicity and poor tolerance late in the course of treatment suggestive of a cumulative effect, with 3 out of 8 mice displaying massive weight loss 4 days after second and third injections, and 5 out of 8 mice presenting severe cutaneous bleedings following 4 days after the second treatment. In contrast, at 4 mg/kg the mice showed no signs of toxicity from neither DP(25) nor DP(40), with 8 out of 8 mice reaching the humane endpoint of tumor burden.
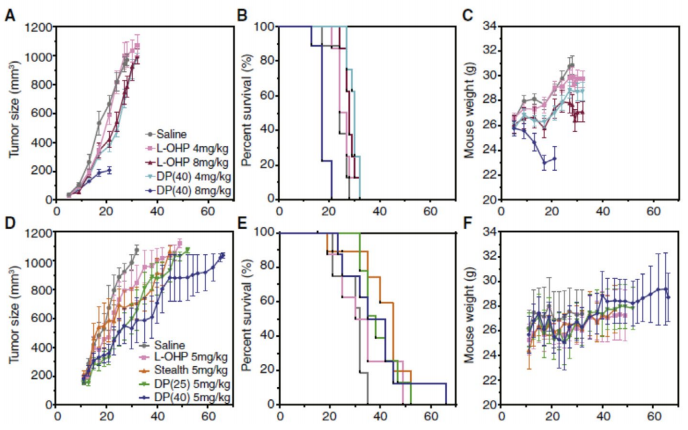 Figure 4. Therapeutic efficacy of sPLA2 -sensitive formulations in female nude NMRI mice bearing FaDu tumors with no sPLA2 expression (upper panel) and Colo205 tumors with high sPLA2 expression (lower panel). In FaDu xenografts, mice received 6 i.v. injections every 4 days (6q4d) of 4 or 8 mg/kg L-OHP free or loaded in highly sPLA2 -sensitive DP(40) liposomes. Colo205-bearing mice received 4 i.v. injections every 4 days (4q4d) of 5 mg/kg L-OHP free or loaded in liposomes with varying degree of sensitivity toward sPLA2 (high, DP(40); low, DP(25); non, stealth). (A, B) Mean tumor volumes. (B, E) Kaplan-Maier survival plots based on specified humane end points. (C, F) Animal body weights.
Figure 4. Therapeutic efficacy of sPLA2 -sensitive formulations in female nude NMRI mice bearing FaDu tumors with no sPLA2 expression (upper panel) and Colo205 tumors with high sPLA2 expression (lower panel). In FaDu xenografts, mice received 6 i.v. injections every 4 days (6q4d) of 4 or 8 mg/kg L-OHP free or loaded in highly sPLA2 -sensitive DP(40) liposomes. Colo205-bearing mice received 4 i.v. injections every 4 days (4q4d) of 5 mg/kg L-OHP free or loaded in liposomes with varying degree of sensitivity toward sPLA2 (high, DP(40); low, DP(25); non, stealth). (A, B) Mean tumor volumes. (B, E) Kaplan-Maier survival plots based on specified humane end points. (C, F) Animal body weights.Continued expression of sPLA2 by human Colo205 colorectal carcinoma cells following implantation in mice has previously been established4 and was confirmed here.1 This allowed us to use Colo205 xenografts, as a sPLA2 -proficient human cancer model with an elevated tumor expression profile of sPLA2 and sensitivity toward L-OHP were therefore expected to serve as a suitable experimental model to evaluate the in vivo performance of the present enzyme-responsive formulations. Here we determined the efficacy of 4 i.v. injections of L-OHP in mice implanted with ectopic Colo205 tumors at 5 mg/kg administered every 4 days, as either unencapsulated drug or formulated in liposomes with a high degree of sPLA2 sensitivity (DP(40)), low sensitivity (DP(25)), and non-sensitive highly stable liposomes (stealth) (Fig. 4D–F). In comparison to FaDu tumors, Colo205 xenografts did exhibit marginally higher response rates toward free L-OHP following three intravenous injections. Although for the liposomal formulations of L-OHP, despite a minor increased growth-inhibiting effect relative to the free drug, encapsulating L-OHP within sPLA2 -sensitive liposomal formulations did not improve the antitumor effect compared with non-sensitive liposomes (Fig. 4D and E). In fact, all of the tested formulations exhibited >45% treatment-to-control ratios (%T/C) and were therefore statistically therapeutically inactive. No significant (P values > 0.05) increase in survival proportions between the different treatments was obtained in the Colo205 model, as were assessed by the median survival of the groups (Fig. 4E). A summary of the parameters from the Colo205 efficacy study is presented herein.1
With respect to the tolerability of the formulations, 2 out of 9 mice bearing Colo205 xenografts receiving four injections of a reduced dose of 5 mg/kg of DP(40) continued to display signs of poor tolerance and experienced excessive weight loss during the course of treatment (4 days after third injection and 2 days after the fourth injection, respectively). The less sensitive DP(25) formulation was on the other hand well-tolerated, with none of the mice displaying systemic toxicity and not accompanied by any weight loss (Fig. 4F). Consistent with the observations in the dose-escalation study, a schedule of multiple injections at a reduced dose of 5 mg/kg (equivalent to a lipid dose of 50 mg/kg for DP(25) and 58 mg/kg for DP(40), respectively) did not cause hemorrhaging in the skin of treated mice. We therefore suspect that the hemorrhages found in the skin are caused by an excessive extravasation of the particles into the skin of the mice when administering multiple large doses, subsequently leading to an activation of the particles by host sPLA2 present in the skin, where it plays an intricate role in maintaining the integrity of the epidermis.5 However, further investigations are needed to address whether or not this diffuse bleeding is also implicated in the mortality of the animals.
These results clearly demonstrate the inability of using the highly sensitive DP(40) formulation at effective tumor growth-inhibiting doses owing to dose-limiting systemic toxicities. Conversely, lowering the sensitivity of the particles toward sPLA2 , as seen with DP(25), the formulation becomes well-tolerated yet is therapeutically inactive and indistinguishable from liposomes not carrying enzyme-triggered release properties. The latter presumably being from a lack in ability to become hydrolyzed inside the tumor to a significant extent.
To establish the underlying cause of toxicity of the DP(40) formulation, histological examination was conducted on the liver of the mice. The principal cause of death was determined to be owing to multifocal peracute hepatonecrotic lesions. Analyzing the liver of a mouse treated with a single high dose of 10 mg/kg of DP(40) showed no signs of liver damage 4 days after treatment (Fig. 5A), whereas following 3 injections of 8 mg/kg every 4 days necrotic lesions became apparent in the liver (Fig. 5B). This illustrates that the cumulative accumulation of the liposomes in the liver causes the toxicity produced in the organ and, thus, to the animal. On the other hand, these signs were not observed in mice treated with low sPLA2 -sensitive (DP(25)) and non-degradable liposomes (stealth) (data not shown), despite it being well-known that PEGylated liposomes do accumulate in the liver to a high extent. Therefore, we believe the liver damage arises from the combined effect of high liver accumulation of the liposomes along with a high sPLA2 degradability. Because elevated levels of this enzyme are produced by hepatocytes,6 we speculate that activation of these particles from the action of hepatic sPLA2 does occur for the DP(40) formulation, resulting in the release of the encapsulated drug, and at the same time high local concentrations of permeability-enhancing components are formed, creating the observed degenerative condition. In addition, the secretion of sPLA2 into the hepatic intercellular space is further exacerbated following drug-mediated injury of the hepatic cells, which can then further deteriorate the breakdown of the tissue in a self-perpetuating and vicious cycle.7
Taken together, these data illustrate that sPLA2 -mediated controlled release from liposomal drug carriers, investigated here for delivering the platinum drug L-OHP, appears to preclinically have a self-limiting effect on the antitumor efficacy by causing inadvertent liver toxicity following systemic administration. The main limitation of this system, therefore, is the release of membraneactive lysophospholipids and free fatty acids driven by hepatic enzymatic hydrolysis, which in particular affects the usage of highly sensitive liposome formulations designed to facilitate fast local drug release inside tumors but more so hampers the dosage required to attain therapeutic efficacy.
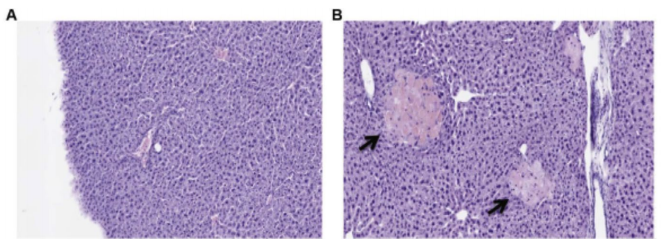 Figure 5. Histological examination of liver after single or multiple dosings of highly sPLA2 - sensitive liposomal L-OHP. (A) No damaged regions were observed in the liver 4 days after a single injection of DP(40) at 10 mg/kg. (B) Necrotic manifestations (arrows) in the liver following three injections of 8 mg/kg of DP(40) administered every 4 days. Multiple injections of high-sPLA2 -sensitive DP(40) formulations result in multifocal peracute hepatonecrotic lesions. Staining was performed with hematoxylin and eosin.
Figure 5. Histological examination of liver after single or multiple dosings of highly sPLA2 - sensitive liposomal L-OHP. (A) No damaged regions were observed in the liver 4 days after a single injection of DP(40) at 10 mg/kg. (B) Necrotic manifestations (arrows) in the liver following three injections of 8 mg/kg of DP(40) administered every 4 days. Multiple injections of high-sPLA2 -sensitive DP(40) formulations result in multifocal peracute hepatonecrotic lesions. Staining was performed with hematoxylin and eosin.In conclusion, we did not find evidence that increased drug efficacy could be obtained by using a sPLA2 -triggered drug release mechanism with high sensitivity, compared with low- or nonsensitive liposomes. In contrast, our findings suggest that sPLA2 -responsive liposomes instead pose a risk of systemic toxicity from a combined effect of both their sensitivity toward the enzyme and the additive up-concentration of dosed particles accumulating in the liver following repeated dosage. Increasing the amount of PG in the liposome membranes to 40% in order to reach a high rate of drug release also increased the incidence of systemic toxicity and restricted their use for in vivo applications, whereas with 25% PG, despite no detectable systemic toxicity in our preclinical model did not produce higher growth inhibition relative to non-degradable PEGylated liposomes.
Overall, the sPLA2 -induced drug release strategy suffers from a narrow therapeutic window, in which sPLA2 -sensitive liposomes are limited by drug-related liver failure, whereas low-sensitive formulations, such as the clinically used LiPlaCis®, lack an actively sPLA2 - controlled released mechanism, thus behaving therapeutically as conventional non-degradable PEGylated formulations. This raises concerns as to whether LiPlaCis® and similar formulations using sPLA2 as a target can benefit cancer patients.
References
1. Pourhassan, H, Clergeaud, G, Hansen, AE, Østrem, RG, Fliedner, FP, Melander, F, Nielsen, OL, Sullivan, CKO, Kjær, A, Andresen, TL. Revisiting the use of sPLA2 -sensitive liposomes in cancer therapy. J. Controlled Release 261: 163-173 (2017). 2. Kim, YL, Im, YJ, Ha, NC, Im, DS. Albumin inhibits cytotoxic activity of lysophosphatidylcholine by direct binding. Prostaglandins Other Lipid Mediat. 83: 130-138 (2007). 3. Kennedy, BP, Payette, P, Mudgett, J, Vadas, P, Pruzanski, W, Kwan, M, Tang, C, Rancourt, DE, Cromlish, WA. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 270: 22378-22385 (1995). 4. Jensen, SS, Andresen, TL, Davidsen, J, Høyrup, P, Shnyder, SD, Bibby, MC, Gill, JH, Jørgensen, K. Secretory phospholipase A(2) as a tumorspecific trigger for targeted delivery of a novel class of liposomal prodrug anticancer etherlipids. Mol. Cancer Ther. 3: 1451-1458 (2004). 5. Gurrieri, S, Fürstenberger, G, Schadow, A, Haas, U, Singer, AG, Ghomashchi, F, Pfeilschifter, J, Lambeau, G, Gelb, MH, Kaszkin, M. Differentiation-dependent regulation of secreted phospholipases A2 in murine epidermis. J. Invest. Dermatol. 121: 156-164 (2003). 6. Crowl, RM, Stoller, TJ, Conroy, RR, Stoner, CR. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J. Biol. Chem. 266: 2647-2651 (1991). 7. Ito, M, Ishikawa, Y, Kiguchi, H, Komiyama, K, Murakami, M, Kudo, I, Akasaka, Y, Ishii, T. Distribution of type V secretory phospholipase A2 expression in human hepatocytes damaged by liver disease. J. Gastroenterol. Hepatol. 19: 1140-1149 (2004).
a Department of Micro- and Nanotechnology, Technical University of Denmark, Building 423, DK-2800 Kgs. Lyngby, Denmark. b Centre for Nanomedicine and Theranostics, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark. c Authors contributed equally to this work. d Department of Clinical Physiology, Nuclear Medicine and PET, Rigshospitalet, Copenhagen University Hospital and Cluster for Molecular Imaging, Faculty of Health Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark. e Department of Veterinary and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Frederiksberg C, Denmark. f Nanobiotechnology and Bioanalysis Group, Department of Chemical Engineering, University of Rovira I Virgili, Tarragona, Spain. g Institució Catalana de Recerca i Estudis Avançats, Barcelona, Spain. h Corresponding author. E-mail: tlan@nanotech.dtu.dk Adapted from Pourhassan et al. (https://doi.org/10.1016/j.jconrel.2017.06.024) 1 with permission from Elsevier.


