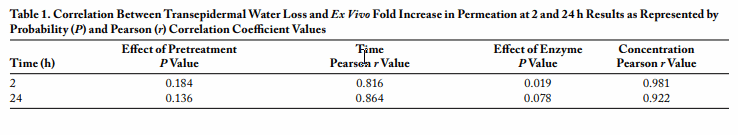
Introduction Microorganisms are contributing to a great degree nowadays in the field of biotechnology, with increasing demand as they provide a wide range of by-products that show promising economic importance and value in many specialized branches of biotechnology such as the food industry, leather industry, waste management, detergent industry, and medical and pharmaceutical applications.1,2 Microbial enzymes offer substantial and increasingly important advantages over chemical catalysts in several ways: they are derived from renewable resources, are biodegradable, work under relatively mild conditions of temperature and pH, and tend to offer exquisite selectivity in both reactant and product stereochemistry.
Alkaline protease enzymes are produced from recombinant bacteria, in a high yield. The enzymes can be explored for various industrial and biomedical applications. The aim of the present work was to investigate the effect of keratinase enzyme, an alkaline protease, on the structural integrity of the skin to provide information necessary for various medical and pharmaceutical applications.
Experimental Methods
Ex Vivo Skin Permeation Studies. Rhodamine B permeation across rabbit ear skin was assessed in vitro at 32 ± 0.5°C using Franz diffusion cells. The skin samples were pretreated with the enzyme. This was followed by permeation of the dye for 2 and 24 h at 32 ± 0.5°C. The effect of enzyme concentration in terms of enzyme units (5, 10, 20, 25, and 30 units) applied to the skin and enzyme pretreatment time (15, 30, 45, and 60 min) as experimental variables was investigated.
Transepidermal Water Loss (TEWL) Measurements. TEWL was determined ex vivo in a separate experiment as reported elsewhere.3 Skin samples were mounted in vertical Franz diffusion cells (PermeGear, Bethlehem, PA, U.S.A.). The receiver phase was 12.2 mL of phosphate-buffered saline (PBS), pH 7.4, stirred at 400 rpm at 32 ± 0.5°C. TEWL was measured using an AF103 AquaFlux device (Biox Systems, London, U.K.) inserted into an empty donor cell secured over each skin sample.
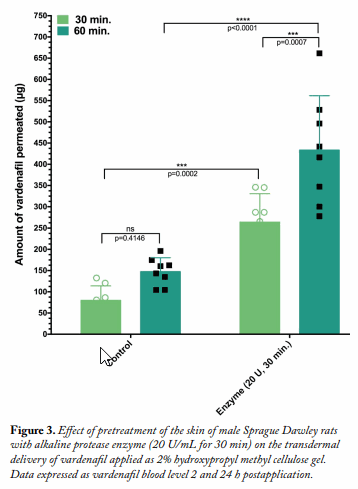
Transdermal Delivery of Vardenafil in a Rat Model. The effect of enzyme pretreatment on the transdermal delivery of vardenafil as model drug applied in a 2% hydroxypropyl methyl cellulose (HPMC) gel vehicle was investigated using Sprague Dawley rats (120–140 g, n = 6). A 1 mL sample of PBS, pH 7.4, containing 20 units of alkaline protease was used to pretreat the marked skin application area for 30 min. Afterward, the enzyme was wiped off, and the application area was cleaned with distilled water. Post enzyme pretreatment, 500 μL of the 2% vardenafil gel was applied to the 3 cm2 circular area.
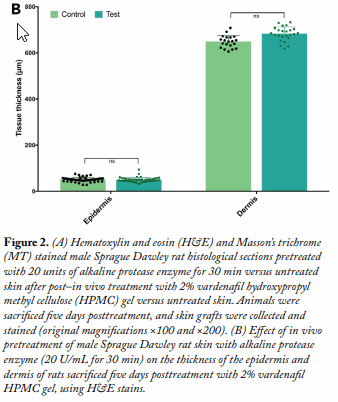
Histopathological Examination and Morphometric Measurements of Skin Samples. This was conducted on both rabbit ear pinna skin samples used in the ex vivo permeation study and dorsal skin, posterior to the scapulae grafted out of the Sprague Dawley rats used in the in vivo permeation study after sacrificing the rats five days subsequent to the permeation study. Test and control skin samples were fixed in 10% formalin and stained with hematoxylin and eosin (H&E) and Masson’s trichrome stains.
Results and Discussion Alkaline protease pretreatment of rabbit ear skin resulted in a significant increase in rhodamine B permeation, the effect being more pronounced at the longer permeation time (24 h). The fold increase in dye permeation reached its maximum at 20 enzyme units and 30 min pretreatment time (Fig. 1A and B). The in vitro skin permeation studies were confirmed via TEWL analysis (Fig. 1C and D, Table 1). Histopathological examination of enzyme-treated skin, along with TEWL data, indicated obvious proteolytic and keratinolytic effects of the enzyme that were dependent on enzyme activity (units) and pretreatment time (Fig. 2).
a Department of Pharmaceutical Sciences, Appalachian College of Pharmacy, Oakwood, VA 24631, U.S.A.
b Department of Pharmaceutics, Faculty of Pharmacy, Alexandria University, Alexandria, 21521, Egypt.
c Corresponding author. E-mail: nounou@acp.edu
d Department of Biotechnology, Institute of Graduate Studies and Research (IGSR), Alexandria University, Alexandria, 21526, Egypt.
e Department of Dermatology, Faculty of Medicine, Alexandria University, Alexandria, 21521, Egypt.
Furthermore, treatment with the enzyme significantly enhanced the transdermal delivery of varde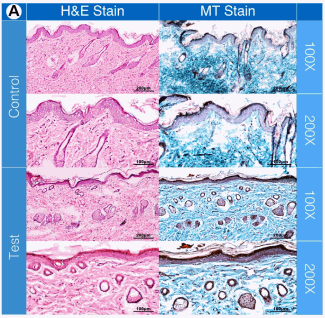 nafil, as indicated by the drug levels in the rats’ plasma 30 and 60 min postapplication (Fig. 3).
nafil, as indicated by the drug levels in the rats’ plasma 30 and 60 min postapplication (Fig. 3).
Conclusions
Data obtained on the effect of alkaline protease on skin structure and function indicated controllable enzyme-induced skin permeabilization. Transdermal drug delivery data provided a proof of concept for a new biological skin permeation enhancement approach with minimal skin damage. The study outcomes coupled with the economic aspects of enzyme production through recombinant bacteria offer great promise as a basis for diverse biomedical and pharmaceutical skin applications. These may include skin permeation enhancement in dermal and transdermal drug delivery, wound debridement, and treatment of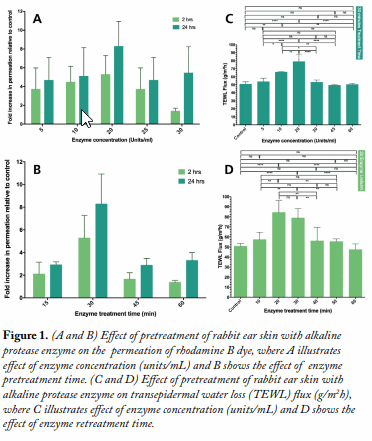
 hyperkeratinization skin conditions. Further investigations are encouraged to transform this promising biological tool, the keratinase proteolytic enzyme, from bench to bedside in pharmaceutical topical applications via optimized formulation parameters and tailored dosage form design. Finally, we believe that cheap, effective, reproducible, and easily scalable industrial biological products, represented by alkaline protease enzyme, have a huge potential in creating successful topical pharmaceutical formulations.
hyperkeratinization skin conditions. Further investigations are encouraged to transform this promising biological tool, the keratinase proteolytic enzyme, from bench to bedside in pharmaceutical topical applications via optimized formulation parameters and tailored dosage form design. Finally, we believe that cheap, effective, reproducible, and easily scalable industrial biological products, represented by alkaline protease enzyme, have a huge potential in creating successful topical pharmaceutical formulations.
References
1. Sukan, A, Roy, I, Keshavarz, T. Dual production of biopolymers from bacteria. Carbohydr. Polym. 126:47-51, 2015.
2. Volmer, J, Schmid, A, Buhler, B. Guiding bioprocess design by microbial ecology. Curr. Opin. Microbiol. 25:25-32, 2015.
3. Gomaa, YA, El-Khordagui, LK, Garland, MJ, Donnelly, RF, McInnes, F, Meidan, VM. Effect of microneedle treatment on the skin permeation of a nanoencapsulated dye. J. Pharm. Pharmacol. 64(11):1592- 1602, 2012. n


