Introduction
Nanoparticulate drug delivery systems offer a huge potential for various biomedical applications, and the number of related research projects has been increasing constantly over the last decade.1The delivery of small molecules, proteins, or nucleic acids to target cells using nanocarriers has several advantages compared with conventional therapeutics. Therefore, the examination of cellular uptake and intracellular trafficking of nanocarriers has become an important research field with great implications for the therapeutic outcome of nanomedicines.2,3Encapsulation of electron dense nanoparticles into nanocarriers is an interesting option for bioimaging using elec- tron microscopy and therefore examination of subcellular localization. Gold nanoparticles (AuNPs) especially have attracted great at- tention owing to their unique properties including (1) advantageous physicochemical characteristics, (2) nontoxic and inert properties,
(3) facile preparation of monodisperse AuNPs, and (4) various modification options.4 In recent decades, great progress has been made in the field of hybrid nanocarriers using AuNPs. However, the formation approaches exhibited marked variability in homogeneity, re- producibility, size distribution, and morphology of hybrid nanocarriers (i.e., nanocarriers encapsulating AuNPs [AuNHyb]). To over- come these challenges, we developed a novel and versatile strategy to encapsulate AuNPs into different nanocarriers with high repro- ducibility using a “nanoreactor approach.” In this study, we encapsulated a reaction solution (tetrachloroaurate/citrate mixture) within the interior of nanocarriers and initiated the AuNP formation after self-assembly of the nanomaterial. Different nanomaterials such as lipids or polymers were validated, and the encapsulation efficiency, homogeneity, and robustness of our approach were optimized. In- tracellular trafficking of these hybrid nanocarriers in vitro was performed using transmission electron microscopy (TEM).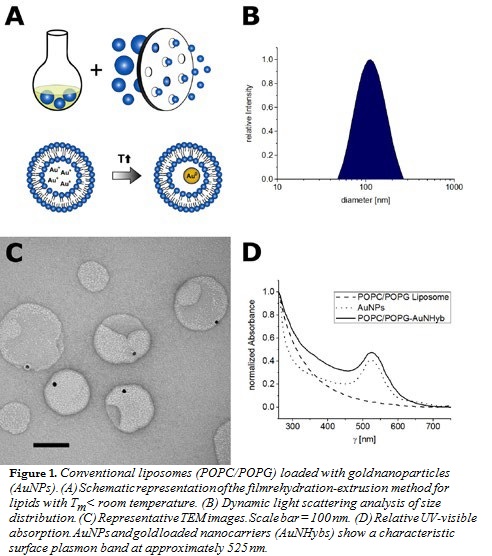
Experimental Methods
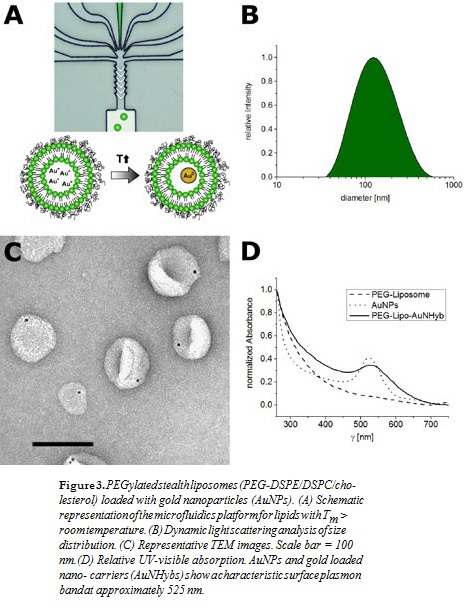
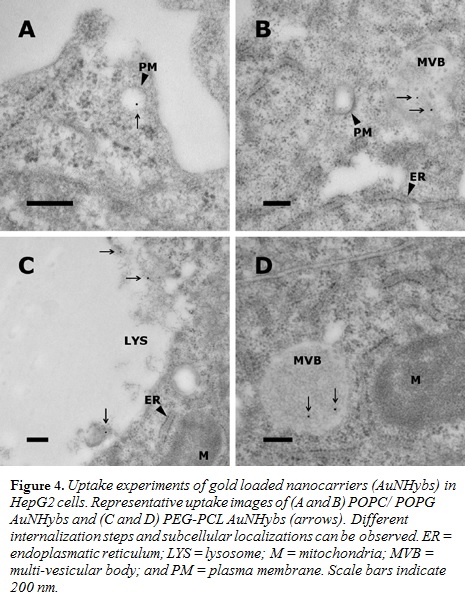
Various nanocarriers such as conventional liposomes (POPC/POPG; Figure 1), di-block copolymer nanoparticles (PEG-PCL; Figure 2), or PEGylated stealth liposomes (PEG-DSPE/DSPC/cholesterol; Figure 3) were produced using the filmrehydration-extrusion, nanoprecipitation, or microfluidics method, respectively. After the self-assembly process the different nanocarriers encapsulated a reaction solution consisting of tetrachloroaurate and citrate. A temperature shift to 70°C initiated the formation of AuNPs inside the nanocarriers. To remove nonencapsulated AuNPs, size-exclusion chromatography was performed. The physicochem- ical characteristics of our lipid and polymer AuNHybs were evaluated using dynamic light scattering (DLS), TEM, CryoTEM, and UVvisible spectroscopy. To examine the application of AuNHybs as a bioimaging tool, we performed cellular uptake experiments in HepG2 cells and used the electron density of AuNPs for further TEM analysis (Figure 4). For detailed experimental procedures, see Witzigmann et al.5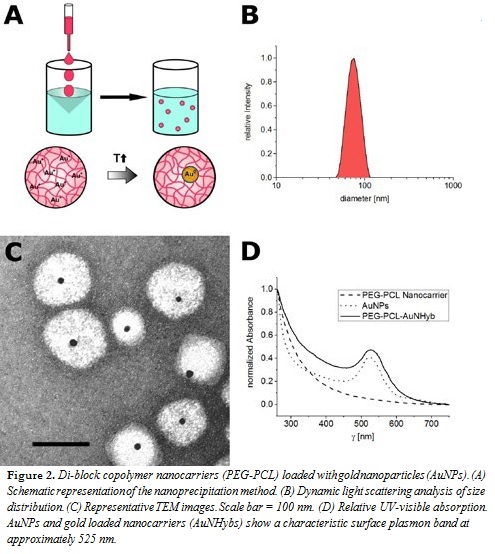
Results and Discussion
For the preparation of AuNP-loaded nanocarriers, the most direct ap- proach is the use of presynthesized AuNPs. However, the encapsulation of preformed AuNPs has several issues, including low encapsulation efficien- cy (data not shown). Therefore, we developed an alternative strategy and combined a variety of preparation methods with our nanoreactor approach (Figures 1–3, panel A). The formation of AuNPs inside the nanocarriers was initiated after self-assembly by a temperature shift. The most impor- tant factor of our approach was the fast production of nanocarriers at room temperature (RT) to avoid the formation of AuNPs before self-assembly.
The film rehydration method is suitable for lipids with a transition tem- perature below RT (Figure 1), whereas the nanoprecipitation method is suitable for various polymer nanomaterials (Figure 2). The microfluidics method is especially designed for lipids with a transition temperature above RT, because the film rehydration method above RT is not compat- ible with the nanoreactor approach (Figure 3). In general, microfluidics is suitable for all nanomaterials that need highly controlled nanomanufac- turing. In addition, this technique offers the possibility for efficient and large-scale production.
In contrast to other methods for AuNP encapsulation, we achieved a high homogeneity and produced nanocarriers with a monodisperse size distri- bution (polydispersity index < 0.2) (Figures 1–3, panel B). The hydrody- namic diameter of AuNHybs determined by DLS was between 50 nm and 120 nm, which is in the size range of nanocarriers for biomedical ap- plications. The AuNPs were encapsulated within the AuNHybs, as clearly visualized by electron microscopy (Figures 1–3, panel C). All AuNHybs exhibited a characteristic ruby-red color resulting from the surface plas- mon resonance of incorporated AuNPs (Figures 1–3, panel D).
Passive uptake experiments of AuNHybs into HepG2 cells demonstrated the applicability as a bioimaging tool (Figure 4). AuNHybs could be used to examine nanoparticle-cell interactions and the intracellular fate of nanocarriers by TEM, allowing a considerably higher resolution compared with confocal laser scanning microscopy. However, if needed, AuNHybs could be combined with fluorescent dyes to offer additional possibilities such as live cell imaging (data not shown).
Conclusion
In conclusion, we have developed a novel AuNP encapsulation technique for lipid and polymer-based nanocarriers. Via this nanoreactor approach, a high AuNP encapsulation efficiency was achieved. To the best of our knowledge, this study is unique in applying
different nanomaterials in combination with various preparation methods. The high reproducibility and versatility of our nanoreactor approach is unprecedented and makes this technology suitable for many nanomaterials. In the future, our nanoreactor approach will be instrumental to develop a better understanding of cellular uptake and intracellular trafficking of nanocarriers.
Acknowledgements
Adapted from Witzigmann et al.5with permission from The Royal Society of Chemistry. Financial support of the “Stiftung zur Förderung des pharmazeutischen Nachwuchses” in Basel is acknowledged. In addition, we thank Prof. Henning Stahlberg (Director, Centre for Cellular Imaging and NanoAnalytics [C-CINA]) for his support.
References
- Wicki, A, Witzigmann, D, Balasubramanian, V, Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications, J. Controlled Release 200:138-157 (2015). doi:10.1016/j.jconrel.2014.12.030.
- Sahay, G, Alakhova, DY, Kabanov, AV. Endocytosis of nanomedicines, J. Controlled Release 145:182-195 (2010). doi:10.1016/j.jconrel.2010.01.036.
- Kettiger, H, Schipanski, A, Wick, P, Huwyler, J. Engineered nanomaterial uptake and tissue distribution: From cell to organism, Int. J. Nanomed. 8:3255-3269 (2013). doi:10.2147/IJN.S49770.
- Pissuwan, D, Niidome, T, Cortie, MB. The forthcoming applications of gold nanoparticles in drug= and gene delivery systems, J. Controlled Release 149:65-71 (2011). doi:10.1016/j.jconrel.2009.12.006.
- Witzigmann, D, Sieber, S, Porta, F, Grossen, P, Bieri, A, Strelnikova, N, Pfohl, T, Prescianotto-Baschong, C, Huwyler, J. Formation of lipid and polymer based gold nanohybrids using a nanoreactor approach, RSC Adv. 5:74320-74328 (2015). doi:10.1039/C5RA13967H. n
aCorresponding author. E-mail: dominik.witzigmann@unibas.ch
Adapted from Witzigmann et al. (http://dx.doi.org/10.1039/C5RA13967H)5 with permission from The Royal Society of Chemistry.


