Introduction
The potent antioxidative effect of flavonoids can be used in cosmetic products to prevent skin from aging and wrinkles, provided a sufficiently high skin penetration can be obtained by the dermal formulation. Cosmetic products can be gel, cream, or lotion. However, the poor water solubility of this kind of active will limit the application because of the consequently low skin penetration and absorption. Nanocrystals for increasing the oral bioavailability are meanwhile also dermally applied to improve the penetration into the skin. Cosmetic products have been on the market since 2007 (for example, Juvena in the Juvedical line since 2007 and Platinum Rare cream by La Prairie since 2009).
The improvement of the skin penetration of poorly soluble actives by nanocrystals is caused by three effects:
- The increased saturation solubility of the active (in terms of the Kelvin equation) generates a higher concentration of the active in the formulation on the skin and, subsequently, a higher concentration gradient between the formulation and skin.1
- The nanosized particles have a high contact area to the skin, are adhesive, and have a long residence time.2
- The small size leads to follicular accumulation of the nanocrystals, promoting absorption and forming a depot.2
Two main methods, bead milling and high-pressure homogenization, can be used in production of nanocrystals on a large industrial scale. Bead milling has been widely applied in the pharmaceutical industry (e.g., Nanosystems/Élan, now part of
Alkermes, U.S.A.), being a low-energy process. High-pressure homogenization was developed as an alternative process in the 1990s to produce nanocrystals with high energy input, allowing also aseptic production (SkyePharma, U.K.; Baxter, U.S.A.).
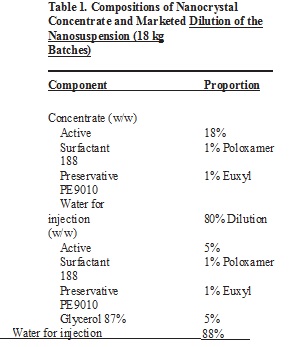
The newly developed smartCrystal® technology combines bead milling with high-pressure homogenization (PharmaSol, Germany).3,4 In this study hesperidin nanocrystals were produced on a large scale as commercial nanocrystal concentrate for incorporation into cosmetic products. Highly concentrated nanosuspension was produced by bead milling first. This intermediate product was then diluted to the final market product concentration (5%) and processed by high-pressure homogenization.
Experimental Methods
Coarse suspension (batch size 18 kg) of hesperidin (Table 1) as the intermediate concentrate was passed five times through a Bühler PML-2 bead mill (Bühler, Switzerland) with a 1,050 mL milling chamber. Yttrium oxide stabilized zircon oxide beads of 0.4–0.6 mm diameter (Hosokawa Alpine, Germany) were used as the milling medium. Milling rotator speed was 2,000 rpm, and pump capacity was 10%. During the production, the milling chamber was cooled at 5°C. The size of nanocrystals was monitored during the milling after each passage. The milled product obtained after five passages was diluted (Table 1) and homogenized with an Avestin C50 homogenizer (Avestin, Canada), applying one cycle at 500 bar to obtain the final market product (Figure 1).
Contents of active and preservative in the final product were measured by HPLC. Size analysis was carried out by photon
 correlation spectroscopy (PCS) (Zetasizer Nano ZS, Malvern Instruments, U.K.), laser diffractometry (LD) (Mastersizer 2000, Malvern Instruments), and light microscopy (Ortoplan, Germany).
correlation spectroscopy (PCS) (Zetasizer Nano ZS, Malvern Instruments, U.K.), laser diffractometry (LD) (Mastersizer 2000, Malvern Instruments), and light microscopy (Ortoplan, Germany).
Results and Discussion
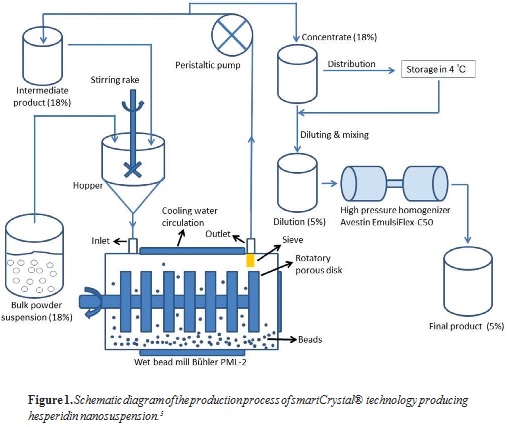
Bead milling led to a steady decrease of the size after each passage (from 1 to 5, Figure 2). After only one passage, the PCS diameter was decreased to 539 nm. However, the existence of large particles was detected by LD, as the diameter d(v)50% was still 1.748 μm. After the fifth passage, the PCS diameter decreased to around 290 nm with a polydispersity index (PdI) of
0.237. The decrease of median LD diameter d(v)50% from passage 2 to passage 5 was minor, but the d(v)90% became lower than 1.5 μm (Figure 2). This result indicates a very small remaining fraction of larger particles in the intermediate product, which would be unproblematic for a dermal product.
 The intermediate product was diluted to 5% hesperidin and processed by high-pressure homogenization. PCS size of the final market product was 265 nm with an LD diameter d(v)99% less than 4 μm (before this step, 290 nm and 4.205 μm). This decrease in size proved that the subsequent high-pressure homogenization could generate further size reduction. In addition, the size distribution was more homogenous owing to the removal of larger particles and aggregates.
The intermediate product was diluted to 5% hesperidin and processed by high-pressure homogenization. PCS size of the final market product was 265 nm with an LD diameter d(v)99% less than 4 μm (before this step, 290 nm and 4.205 μm). This decrease in size proved that the subsequent high-pressure homogenization could generate further size reduction. In addition, the size distribution was more homogenous owing to the removal of larger particles and aggregates.
The chemical contents of hesperidin and Euxyl PE 9010 were
4.9 and 1.0%, respectively, which was nearly identical to the theoretical content and was highly acceptable considering typical variations in large-scale production.
Conclusions
Processing parameters for a large-scale production process for hesperidin nanocrystals were established based on the smartCrystal® technology. The batch size of 18 kg of intermediate concentrate (which corresponds to 65 kg of marketed product) can be increased by simply multiplying the volume of suspension passing the bead mill during each passage. The process with the Bühler PML-2 bead mill can be run cost-effectively and can be fully automated 24 h a day. By combining bead milling and high- pressure homogenization, a product with increased physical stability is obtained compared with using only one of the milling processes.3 This observation is of interest for dermal products, which often contain electrolyte-type ingredients, impairing the physical stability by zeta potential reduction.
Acknowledgements
The authors thank PharmaSol GmbH in Berlin for R&D support.
References
- Junghanns, JU, Müller, RH. Nanocrystal technology, drug delivery and clinical applications, Int. J. Nanomed. 3(3): 295-309 (2008).
- Müller, RH, Gohla, S, Keck, CM. State of the art of nanocrystals— Special features, production, nanotoxicology aspects and intracellular delivery, Eur. J. Pharm. Biopharm. 78(1): 1-9 (2011).
- Petersen, R. Nanocrystals for use in topical cosmetic formulations and method of production thereof. Patent application WO 2008/058755 A1 (2008).
- Müller, RH, Chen, R, Keck, CM. smartCrystals for consumer care & cosmetics: Enhanced dermal delivery of poorly soluble plant actives, Household Pers. Care Today 8(5): 18-23 (2013).
- Chen, R. Tailor-made antioxidative nanocrystals: Production and in vitro efficacy, Ph.D. thesis, Freie Universität Berlin, Germany (2013). n


