Separation Done Right: Selection and Validation of the Best Dissolution Filter
Choosing an appropriate dissolution filter is one of the most important aspects of a dissolution method. Filtration is also, however, easy to misunderstand. Many analysts focus on filters as being a way to “clean” the samples for proper analysis by HPLC or UV, but filters play a more important role in the dissolution test than may be initially assumed.
Why are filters important?
Filters are a critical component of the dissolution method for two main reasons. The first, and what most people think of, is to prevent large particles from traveling through automated systems, HPLCs, and other types of flow paths which can cause clogging and cleaning issues. Many analysts will choose a very small pore size filter, particularly if the sample will be run on an HPLC/UPLC. This is important, but often is not critical given the small sample volume being injected.
The second job that a filter has, and the most important part of the test, is to stop the dissolution process. When an experiment is occurring, there will be a mixture of solid particles traveling through the vessel. The filter must be able to screen out the undissolved drug particles as well as any excipients. If undissolved drug can pass through a filter, it will dissolve in the sample that has been collected and create a biased result. These results will inevitably be higher and more variable than a properly filtered sample. What is most commonly seen when a filter with too high a pore size—or no filter—is used is an unexplainably high amount of spikes in the resulting data. When there is a large undissolved drug particle dissolve in the relatively small volume of the sampling lines, test tube, or HPLC vial, this may cause an unexpectedly high result that becomes difficult to explain, especially if the next timepoint returns to normal parameters once the particulate has been flushed out.
Choosing the right filter will result in a clean sample that is an accurate representation of the snapshot in time when it was taken.
Choosing the Right Dissolution Filter
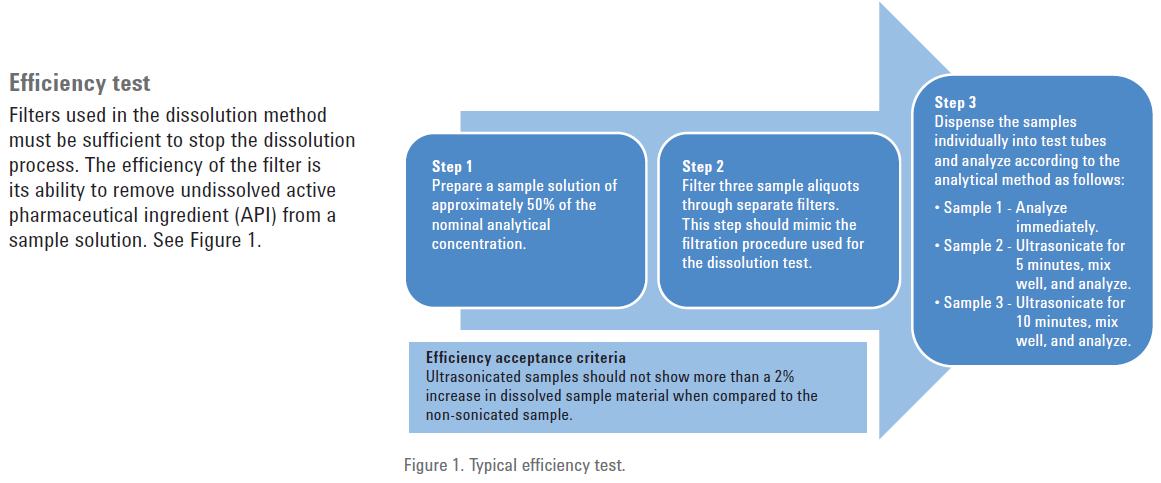
Dissolution filters must be properly validated to ensure that they do not create a biased sample. Filters must be evaluated for three parameters: efficiency, adsorption, and leachability.
Efficiency is a measure of how well the filter can screen out undissolved drug particles. This can be tested by taking aliquots of the same dissolution sample which is partially dissolved and testing the aliquots at different times. The first aliquot should be read immediately, the second after five minutes—typically with some shaking or sonication—and the third after 10 minutes. If a rise of more than 1% of the dissolved value does not appear, then the efficient filter is efficient. If this test does not pass, a smaller pore size filter will need to be selected.
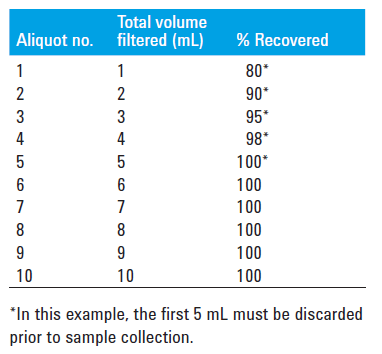
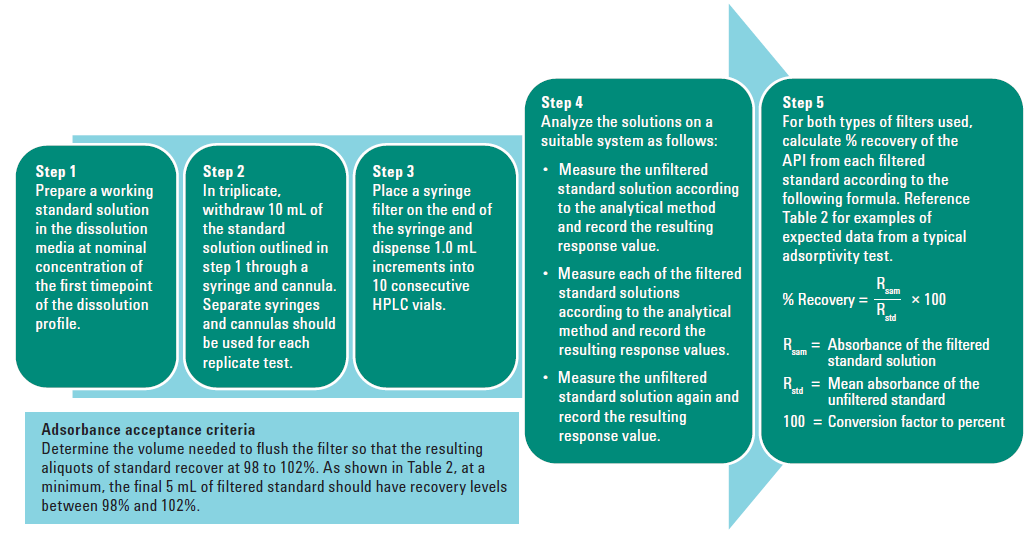
Adsorption is the measure of how much drug is retained on the filter. If a filter holds onto too much of our drug, it can result with a sample of lower concentration than the solution that was pulled from the dissolution vessel. All filters will have some drug binding which needs to be evaluated. Filter adsorption can be evaluated by preparing and sampling from a standard solution at a concentration equivalent to the first timepoint – 1 mL at a time, dispensed into separate HPLC vials or test tubes. What this will usually result in is the first 1-3 mL showing a significant decrease in concentration, and then as the active sites on the filter are bound up the appropriate concentration will be reached. These filtered samples should be compared to an unfiltered sample of the same standard. If adsorption is found, discarding the appropriate volume to waste where there is binding is acceptable, or a different chemistry filter can be evaluated.
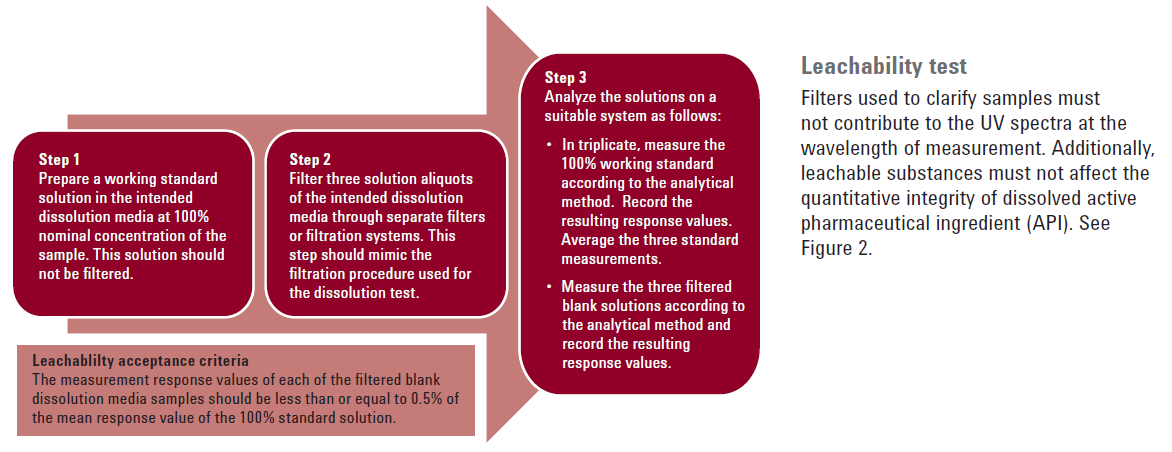
Leachability is checking that the filter itself isn't bleeding any dyes or chemicals into the sample, which can interfere with proper analysis. This can be easily checked by sampling the dissolution media with a filter and comparing a scan of that filtered blank to the standard.
Having a filter that properly meets these three criteria is essential to a proper dissolution test.
What Types of Filters Are Available?
There are a variety of dissolution filters available depending on the properties of the product. Cannula filters, syringe filers, and hollow fiber filters (NanoDis) are all possible options.
Cannula filters (e.g., Agilent Full Flow filters) are most commonly used for dissolution samples. These filters are available from 1 – 70 µm and are relatively inert, making them a suitable choice for most methods. These filters provide filtration at the point where the sampling occurs, which prevents drug particles from dissolving in the cannulas, syringes, and tubing, etc. Even in cases where finer filtration is needed, it is advisable to use a cannula filter as an initial filtration step to prevent contamination of the sample flow path and to help avoid overloading the finer filter. This type of filter is typically utilized for the entire dissolution run due to their high surface area and pore size.
|
Did you know that there are two distinct types of cannula filters?
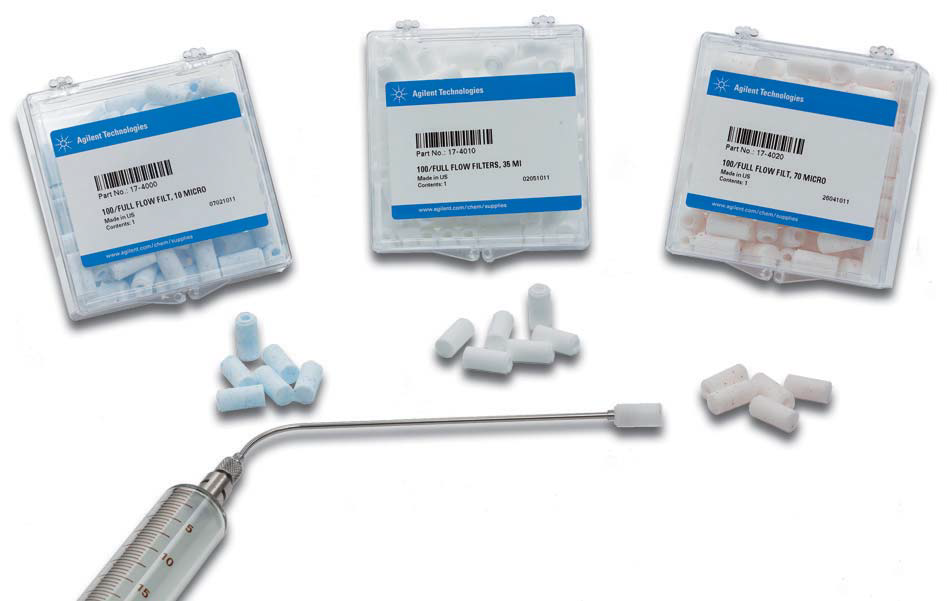
Full flow filters have a higher surface area for filtration and pull the sample from all around the filter tip This allows them to be more efficient, have a higher capacity for removing undissolved material, and less likely to become blocked or clogged. |
Syringe filters can be used where filtration is required at a lower pore size than possible with a cannula filter. These filters are available in sizes from 0.22µm – 1µm and can be used manually or in semi-automated or online UV-Dissolution systems, utilizing the Agilent 850-DS Sampling Station or Agilent Cary 60 UV-Vis spectrophotometer. Syringe filters have more limited surface area and smaller pore size, which means they have a lower capacity for removing undissolved material than cannula filters and are more likely to block; they can usually only be used for 1-2 samples before needing to be replaced. These filters also have the possibility of a strong backpressure, so it is imperative to ensure that excessive force isn’t needed to push the sample through the filter. This can result in mechanical issues in automated systems or can even force undissolved drug through the filter leading to incorrect results.
A third option for filtration is hollow fiber filters which are utilized as part of the Agilent NanoDis System. This type of filter is very useful when dealing with particles on the micro- or nanoscale where filtration is needed below 0.22µm, or when there are challenges with capacity, blocking, or backpressure in syringe filters. In this system, filtration occurs perpendicular to the flow of the sample allowing for very fine filtration without backpressure and an increased surface area and capacity for filtrating difficult samples. For nanoparticles, having a low-pressure filtration mechanism is critical to give accurate results in dissolution analysis. The hollow fiber filters can be used throughout the dissolution test without being changed; with proper rinsing and validation, these filters may be utilized for multiple tests. The NanoDis system provides the pumping and software parameter control to enable these filters to be used repeatedly with high confidence in filter performance.

To access more information or continued discussion on the topic of dissolution filtration, we invite you to visit the Agilent Dissolution Community. There you will find a wealth of information and links to contact our in-house experts directly for support.

