By Vicki Ronaldson
Drug Delivery International (DDi) is a formulation development company that specialises in rescuing problem formulations. With extensive experience in the field of controlled release, it has recently developed intellectual property in novel time-delayed oral formulations. The robust tablet technology, developed over the last two years and now at the licensing stage, allows controllable pulsatile release profiles to accommodate almost any drug type. The work was presented at the 2011 CRS Annual Meeting & Exposition in National Harbor, MD, U.S.A. during the soapbox sessions.
DDi’s Chief Scientific Officer, Professor Alex Mullen, talked to Vicki Ronaldson about the advantages of time-delayed systems, how they can address issues such as circadian rhythms, patient compliance, and product lifecycle management, and how DDi addressed these issues during the development process.
Q What problem were you trying to solve?
A The responsiveness of the body to drugs—their absorption, distribution, metabolism, and elimination—has been shown to vary considerably with the circadian cycle, or “body clock.” As such, careful control of the release of drugs at key points in the circadian cycle can be used to increase the therapeutic efficacy and/or decrease the toxicity of a drug. In addition, the severity or occurrence of disease is affected by the circadian cycle. Often (and particularly with disorders that are most prevalent in the middle of the night or very early in the morning), the patient may not be taking the drug at the time when it would give them the most benefit. Hypertension, for example, has been shown to follow a circadian pattern: both heart rate and blood pressure peak early in the morning, and many people with hypertension experience a marked rise in blood pressure upon awakening (known as the “a.m. surge”). By developing a delayed drug release system, it is possible for the patient to take the tablet before bed yet only receive the dose 5–6 hours later so that it is absorbed at the time when it is most needed.
 |
The responsiveness of the body to drugs varies considerably with the circadian cycle, or body clock. |
Q What makes time-delayed formulations an attractive option, and what drug types and patient groups are likely to benefit?
A Time-delayed formulations are the only way to ensure that the drugs are released into the body at the correct time. The technologies developed by DDi can be used for almost all drug types and for any therapeutic indication. The potential applications are vast, from release of drugs in the middle of the night—to treat insomnia, for example—to overcoming compliance issues. An example of such a compliance issue could be children who need to take two doses of medication during their school day. If the child can be administered an immediate-release dose along with a delayed-release formulation in the morning, the parent can be sure that the child is receiving the medication. It also removes any potential stigmas surrounding taking medications at school. So the technology can provide tremendous benefits to all patient groups.
Q What work has already been done in this field, and how did you identify a gap?
A There has been a great deal of work done on controlled drug release, of course, but this has been predominantly based around sustained release over long periods of time. There are very few delayed-release formulations on the market, and this is because the technology required to produce these formulations was very complicated and technically challenging, introducing manufacturing and cost burdens. The technology developed by DDi utilises simple and well-understood manufacturing processes and, as such, makes delayed drug delivery an attractive option.
Q Briefly, how does the system work?
A Our technology is a tablet-within-a-tablet formulation: a core tablet, containing the drug of choice, surrounded by an erodible barrier. The outer barrier has a constant erosion rate that can be tuned to control the time lag prior to release of drug from the core. The rate of erosion has been shown to be independent of pH, stomach fat content, and agitation.
Q Time-delayed release systems can be in the body for several hours before drug release is required. What challenges did this pose in the design stage? What physiological factors were considered?
A Our formulations, unlike other systems, do not rely on pH changes within the gastrointestinal tract, affording more reproducible performance compared with those systems that incorporate a variety of charged polymeric materials to control drug release. This makes the performance of our formulations independent of typical physiological factors and dependent on an erosion mechanism that can be precisely controlled, allowing for reproducible performance.
Q What drug(s) have you applied this technology to, and why did you choose them?
A DDi has carried out proof of concept studies in three therapeutic areas: cardiovascular, sleep maintenance, and pain and morning stiffness associated with arthritis. These were chosen because they are therapeutic areas with a clear clinical need.
For sleep, the target was to develop a delayed-release formulation to tackle sleep maintenance insomnia, a disorder characterised by continual nocturnal wakening. The DDi formulation incorporates a two-hour delay in drug release. So although you take the drug on going to bed, you do not receive the dose until it is needed to prevent wakening.
It is well known that individuals with rheumatoid arthritis suffer significantly with pain and stiffness upon wakening, severely impacting on their normal daily functions. The DDi formulation provides an immediate nighttime release of diclofenac, followed by a seven-hour time delay before pulsatile release of a second dose of the drug. This formulation offers immediate pain relief at nighttime, allowing pain-free sleep, and subsequently provides delivery of pain relief prior to waking.
The cardiovascular formulation was designed to target drug delivery before wake-up. As mentioned, hypertension has been shown to follow a circadian pattern whereby both heart rate and blood pressure peak early in the morning, as well as the commonly occurring a.m. surge described previously. DDi has developed a formulation for the delivery of anti-hypertensive drugs in the middle of the night, prior to wake-up, when the risk of an adverse cardiovascular event such as a fatal heart attack is greatest.
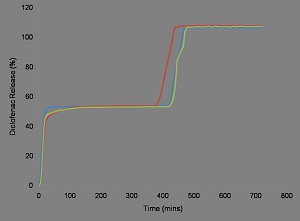 |
In vitro dissolution profile of a dual pulsed release diclofenac formulation designed to address rheumatoid arthritis pain at night and upon waking. |
Q What clinical evaluation has been carried out, and what in vitro work was carried out leading up to this?
A The DDi technology is the result of many years of research; it has been rigorously tested in vitro in dissolution studies and in three proof of concept in vivo trials (for sleep, pain, and cardiovascular), with gamma scintigraphic imaging carried out by our sister company, Bio-Images Research. Each clinical study was performed in six healthy volunteers. In addition to imaging the break-up of the formulation after time delay, pharmacokinetics studies were carried out to confirm that the drugs were being absorbed as anticipated.
Q Was there good in vitro–in vivo correlation?
A The in vitro–in vivo correlation is very strong. The beauty of the system is that it is very robust; it works independently of pH, stomach fat content, and agitation. Our studies have shown that the rate of erosion is constant in vitro and matches that seen in the stomach and in the small intestine.
Q What were the major challenges faced during the development of the product?
A Initially, the major challenges were in getting people to listen to the design concept and securing sufficient funds for the prototypes to be designed and evaluated. We were fortunate in that the Scottish government, through the Scottish Enterprise Proof of Concept scheme, permitted us to realise the value of the formulation concept and to initiate a plan to commercialise the delivery platform.
Q What’s the next stage for this technology? Who will likely benefit from in-licensing it?
A We are continuing to develop the technology through clinical phases and are seeking partners. The DDi delayed delivery system offers huge benefits to all pharmaceutical companies, particularly in lifecycle management. The flexibility and robustness of the formulation means that it can easily be adapted to most drugs, providing opportunities to expand existing product lines but also offering new product development opportunities.
Q What’s the next challenge for DDi in terms of developing improved controlled release technology?
A We are now in discussions with a number of pharmaceutical companies looking to extend the clinical indications of our core technology. In addition, we have been undertaking research and development to design new systems that will offer reproducible oral controlled release that is distinct from other systems on the market.
Those interested in collaborative research opportunities or in licensing of this technology should email enquiries@dd-int.com. For more information on DDi’s activities in formulation development, visit www.dd-int.com.

References
Mullen, AB, Eccleston, SA, Hodges, LA, Ronaldson, VA, Sime, K, Pecek, D, Stevens, HNE. Correlating formulation behaviour with physiological effects for a time-delayed sleep tablet. Controlled Release Soc. Annu. Meet. [Abstr.] 250 (2011).
Mullen, A, Eccleston, S, Hodges, L, Sime, K, Pecek, D, Stevens, H. Controlled-release diclofenac for early morning pain and stiffness. [Abstr.] AAPS J. 13(S2):W5125 (2011).


